Pravachol dosages: 20 mg, 10 mg
Pravachol packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills

Order pravachol from india
Plasma adiponectin levels in newborns are higher than these in adults and positively correlated with start weight. Cord plasma concentrations of adiponectin and leptin in healthy time period neonates: constructive correlation with birthweight and neonatal adiposity. Role of leptin within the regulation of growth and carbohydrate metabolism in the ovine fetus during late gestation. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Glucocorticoids and the preparation for all times after delivery: are there long-term consequences of the life insurance coverage Maternal testosterone levels throughout being pregnant are related to offspring dimension at birth. Free testosterone levels in umbilical-cord blood predict infant head circumference in females. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. Cortisol ranges in umbilical vein and umbilical artery with or without antenatal corticosteroids. Late gestational maternal serum cortisol is inversely associated with fetal brain progress. Maternal cortisol and offspring birthweight: results from a large potential cohort examine. Synthetic glucocorticoid reduces human placental system a transport in ladies handled with antenatal therapy. Regulation of placental amino acid transporter activity by mammalian goal of rapamycin. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell floor abundance in major human trophoblast cells. Mammalian goal of rapamycin within the human placenta regulates leucine transport and is down-regulated in restricted fetal progress. Chen Y-Y, Rosario Fredrick J, Shehab Majida A, Powell Theresa L, Gupta Madhulika B, Jansson T. A novel rat model of gestational diabetes induced by intrauterine programming is related to alterations in placental signaling and fetal overgrowth. Impairment of insulin receptor sign transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal progress. Intrauterine development restriction in people is associated with abnormalities in placental insulin-like growth factor signaling. Effects of gestational diabetes mellitus on proteins implicated in insulin signaling in human placenta. Activated translation signaling in placenta from pregnant women with gestational diabetes mellitus: potential position of leptin. Glucocorticoid-induced fetal origins of adult hypertension: affiliation with epigenetic occasions. Corticosterone alters materno-fetal glucose partitioning and insulin signalling in pregnant mice. Dexamethasone therapy of pregnant F0 mice results in father or mother of origin-specific adjustments in placental operate of the F2 technology. A physiological increase in maternal cortisol alters uteroplacental metabolism within the pregnant ewe. Maternal factors are associated with the expression of placental genes concerned in amino acid metabolism and transport. The impact of a maternal infusion of amino acids on umbilical uptake in pregnancies sophisticated by intrauterine growth restriction. Regulation of amino acid transporters by adenoviral-mediated human insulin-like progress factor-1 in a mouse mannequin of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. These medical merchandise embody drugs from animals, crops, or human origin and products obtained through synthetic pathways, medical devices, and mixture merchandise the 2 classes are as follows: 1) Analytics within the discovery course of (R&D) of pharmaceutical products 2) Analytics within the compliance of merchandise to their requirements in the market Broadly speaking, the primary class is a proactive approach whereas the second category is reactive. The quality, security, and effectiveness of pharmaceutical merchandise are indicated through evaluation of products that act as surrogates for these characteristics.
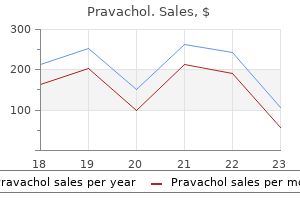
Pravachol 10mg fast delivery
Chloramphenicol, erythromycin (a macrolide), clindamycin (a lincosamide), quinupristin (a streptogramin), dalfopristin (a streptogramin), linezolid (an oxazolidinone), and retapamulin (a pleuromutilin) every inhibit bacterial translation by targeting the 50S ribosomal subunit. Erythromycin also can produce acute cholestatic hepatitis (with ever, jaundice, and impaired liver unction), in all probability as a hypersensitivity reaction. Metabolites o erythromycin can inhibit certain cytochrome P450 isozymes within the liver and thus enhance the plasma focus o numerous medicine which would possibly be also metabolized by these liver enzymes. Azithromycin and clarithromycin are usually nicely tolerated, though these drugs can even cause liver impairment. Low-level resistance has emerged in large chloramphenicol-susceptible populations by the choice o mutants with decreased permeability to the drug. The extra clinically signi cant sort o chloramphenicol resistance has arisen rom the spread o speci c plasmid-encoded acetyltransferases (at least three types o which have been characterized) that inactivate the drug. The undamental mechanism underlying the toxicity o chloramphenicol seems to contain inhibition o mitochondrial protein synthesis. One mani estation o this toxicity is the grey child syndrome, which might occur when chloramphenicol is administered at excessive doses to newborn in ants. Because newborns lack an e ective glucuronic acid conjugation mechanism or the degradation and detoxi cation o chloramphenicol, the drug can accumulate to toxic levels and cause vomiting, f accidity, hypothermia, grey shade, respiratory distress, and metabolic acidosis. More requently, chloramphenicol causes dose-related, reversible despair o erythropoiesis and gastrointestinal misery (nausea, vomiting, and diarrhea). O special interest are the opposed e ects that chloramphenicol may cause in tandem with other medication. Chloramphenicol additionally antagonizes the bactericidal e ects o penicillins and aminoglycosides, as do different bacteriostatic inhibitors o microbial protein synthesis. Mechanism of motion of erythromycin, clindamycin, and chloramphenicol revealed by crystallographic analysis of drug binding to the 50S ribosomal subunit. Clindamycin and macrolides have partially overlapping binding websites on the 50S ribosomal subunit, as do clindamycin and chloramphenicol. The precise binding places and con ormations o the drugs differ in crystal buildings o drug-bound ribosomes rom di erent species. Telithromycin is contraindicated in patients with myasthenia gravis because it exacerbates weakness and can result in respiratory compromise in sufferers with this illness. The most necessary indication or clindamycin is the remedy o critical intra-abdominal or gynecological in ections which are prone to include penicillin-resistant Bacteroides ragilis and other intestinal anaerobes. Clindamycin is a trigger o pseudomembranous colitis attributable to Clostridium di f cile overgrowth. The most extremely vulnerable organisms embody Haemophilus in uenzae, Neisseria meningitidis, and a few strains o Bacteroides. However, the potential or severe toxicity has restricted the systemic use o chloramphenicol. Dal opristin/quinupristin was approved or the therapy o severe or li e-threatening in ections caused by vancomycinresistant Enterococcus aecium or Streptococcus pyogenes. Common opposed e ects o dal opristin/quinupristin include elevated bilirubin, pain with administration, and joint and muscle pain. The A part binds to a location overlapping both the A web site and the P web site in the peptidyl trans erase center, and it may possibly inhibit peptidyl trans erase in vitro. Oxazolidinones resistance to pleuromutilins are in maintaining with this binding site. These compounds inhibit peptide bond ormation, but once elongation is underway and the A and P sites are occupied, pleuromutilins are no longer lively. Adverse e ects o retapamulin are minor and largely limited to local irritation and pruritus on the site o use. The act that three o probably the most recently developed antibiotic classes inhibit the ribosome emphasizes the persevering with value o this complicated construction as a target or new drug development. There is far continuing e ort to uncover new protein synthesis inhibitors, and this work is aided by the availability o structures o ribosomes certain to medication. Several o these antibiotic classes-the quinolones, ri amycin derivatives, f daxomicin, and a ew o the protein synthesis inhibitors- could be bactericidal, but most protein synthesis inhibitors are bacteriostatic.
Diseases
- Rosai Dorfman disease
- Collagen disorder
- Waardenburg syndrome, type 4
- Non-24-hour sleep-wake disorder
- Night blindness
- Transitional cell carcinoma
- Duplication of leg mirror foot
- Hyperphenylalaninemia due to 6-pyruvoyltetrahydrop
Buy pravachol 20mg amex
The public outcry over the unsanitary and unsa e circumstances o the meatpacking trade: this resulted within the Pure Food and Drugs Act in 1906, which prohibited interstate commerce in adulterated and misbranded ood and drugs. The discovery that thalidomide, used to deal with morning illness, brought on birth de ects in large numbers o babies born in Europe: this resulted in passage o the Kefauver-Harris addition to sa ety prior to drug approval and mandated reporting o adverse occasions. It reauthorized prescription drug and medical system user ees and carried out generic drug and biosimilar biologic merchandise user ees. The li e cycle o approval or a brand new drug is advanced, ranging rom 8 to 15 years or completion. The f rst patents are often f led at this stage and are granted several years later. Once a drug is approved, it must be monitored or sa ety or the remainder o its li espan (postmarketing surveillance). Regulatory agencies all over the world have codif ed requirements o moral behavior or all events involved in clinical research, including clinicians, pharmaceutical corporations, and medical establishments. The moral relationship is governed by the notion that clinical trial research represents a partnership between investigator (physician) and subject (volunteer or patient). Four main ethical rules, established by the International Conference on Harmonization and the Declaration of Helsinki, help this partnership. These ideas are as ollows: the trial must decrease the risks or participants. The investigator is responsible or terminating the trial when the dangers become incompatible with the objectives o the trial. Clinical trials have to be appropriately designed and rigorously executed so as to optimize the ratio o benef t to danger and to satis actorily reply the scientif c questions underneath study. Scientif c scientific trial design must include appropriate control or comparator arm(s), randomization and blinding, and pattern measurement, among other parts (see below). Some institutions have a scientif c review committee that must approve all protocols involving human subjects to ensure that the protocol is appropriately designed to answer the questions being asked. During this stage o testing, described in Chapter fifty one, a compound is studied to determine its biological actions, chemical properties, and metabolism, and a course of is developed or its synthesis and purif cation. A main ocus o preclinical testing is determining whether or not the molecule has a suitable sa ety prof le in animals previous to initiating testing in humans. The International Con erence on Harmonization has established requirements or the animal studies used to support di erent sorts o medical trials. As described in Chapter 51, the period o animal research is set by the length o the medical trials to be undertaken. The preclinical analysis section is also an important time to explore potentially necessary pharmacodynamic markers and other biomarkers that could help acilitate scientific improvement. The areas o evaluation embrace a chemistry evaluation, a pharmacology/toxicology review, and a medical evaluation. The sponsor should handle any issues in query be ore the medical hold is li ted. The sponsor could submit further data to assist the sa ety o the proposed trial and a subsequent spherical o sa ety review is initiated. I the sa ety is deemed acceptable, the research may proceed a ter the 30-day evaluation period. The key parts o the goal product prof le include the primary indication, goal affected person population, route o administration, pharmaceutical ormulation, dosing schedule, e f cacy assessments, anticipated major endpoint in pivotal trial(s), anticipated sa ety prof le, and key product traits, including those that might permit di erentiation compared to products already available. The topics may be regular volunteers or patients with specif c ailments; the trials could also be interventional. The key components or consideration when creating any clinical trial are detailed in Table 52-1. Title o research Study hypothesis(es) including strategies to test hypothesis(es) Study aims Study design Indicate any proposed substudies and their design features. Sample measurement Calculated sample size, quantity o websites required, and number o subjects per website. Enrollment period Total projected time or enrollment period, including detailed timing or varied periods. Study drugs (or product) Speci y all (test and comparator) medicines to be used and how they are going to be obtained.
Purchase pravachol overnight
In this text we provide an outline of the explanations that have been proffered for the worth of cancer care, describe a variety of strategies for slowing the rate of growth in the prices of most cancers care in the context of the precision oncology revolution, and eventually define how consideration of the economics of most cancers remedy may influence go/no-go decision making in R&D and health system choice making for brand spanking new precision most cancers therapies. As such, it appears doubtless that other elements should clarify the change within the price of latest cancer drugs. One attainable explanation is that targeted therapies are dramatically simpler than conventional therapies. Although focused therapies do certainly appear to produce bigger medical effects, the magnitude of this improvement is notably less than the observed increase in costs by established worth thresholds for health enchancment. The reduction in the volume of gross sales mixed with high R&D costs would certainly drive up costs if the return on funding is to be maintained for companies. Newer precision most cancers therapies are sometimes biotherapies corresponding to monoclonal antibodies rather than small molecules. The efficiency of the manufacturing of biopharmaceuticals, however, improved hugely between 2001 56 Problem Solving Through Precision Oncology and 2014: the yield increased from 1 to 2. Addressing the fee crisis in precision cancer therapies A range of novel policy responses have been proposed by governments confronted with the necessity to understand the promise of precision drugs whilst containing costs, including increased transparency in R&D costs. Funding is, nevertheless, for a finite interval and will be explicitly dependent upon the collection of additional evidence to inform a definitive funding evaluate at the end of the data collection period. These fall into one of three classes: (1) pay for performance, (2) solely with research, and (3) solely in analysis. Pay-for-performance schemes link the worth that a producer receives to the noticed value of the technology in follow. There is broad variation in the details of the design of pay-forperformance schemes, which might operate either on the individual affected person degree or on the patient population stage. The general precept is that an agreed expected profit is prespecified; initially discounted costs are then topped up or a refund is given, depending on whether benefits are realized or fall brief. Only-with-research schemes make the remedy available for all sufferers with an agreed scientific indication(s), so long as a predefined research research is underway. Hence, focusing on the necessities of the regulatory authorities � for evidence of security and efficacy � has pushed the design of R&D processes. Adding failure at the reimbursement section of translation to the approximately 90% failure fee for candidates getting into phase I scientific Chapter 11: Economic Challenge of Healthcare Provision in Precision Oncology fifty seven trials may symbolize an existential risk to realizing the promise of precision medication. Payers are excited about precise well being achieve once they think about effectiveness, somewhat than organic surrogates of well being corresponding to tumour shrinkage or progression-free survival. A shortfall in evidence for these dimensions will increase uncertainty in an evaluation of worth not mirrored in the standard statistical analysis of medical trials based mostly on hypothesis testing and p-values. Evaluation of tests directing precision therapy There is an extra layer of uncertainty in reimbursement choice making for precision medicine technologies within the analysis of the testing applied sciences that guide remedy. The focus has been on quality assurance of laboratory processes quite than medical outcomes. Tests have been funded by way of hospital laboratory budgets, with choices being made at that budgetary stage. In the context of precision oncology, nonetheless, exams are connected to drug treatments as companion diagnostics, and are becoming subjected to far more detailed analysis in parallel to the companion pharmaceuticals. The analysis of companion diagnostics may have necessary implications for the adoption of precision medicine technologies, and new strategies are required. It is of particular observe that, the place a check offers a steady read-out or rating, the selection of cut-point that defines a constructive or adverse test end result may be defined on grounds of cost-effectiveness along with clinical effectiveness. It might be necessary to find the right implementation of a test�treat combination within an typically complex medical pathway using optimization methods. A wide range of novel reimbursement methods are being employed to manage the challenge. In the longer term, the oncology group might need to think about tips on how to modify R&D processes in recognition of the evolving reimbursement surroundings. The assumption that reaching a licence will be sufficient to ensure affected person access and income is not valid. It is the demonstration of value, in phrases of enhancements in affected person well being, that would be the key to business success. An growing proportion of oncological innovations might be precision drugs applied sciences; due to this fact, oncology will stay on the forefront of efforts by reimbursement authorities to include costs whilst maximizing population health. It shall be essential to understand the impact of their methods on the return on funding for trade as well as on public well being. The final twenty years have seen massive advances in our understanding of the biology of most cancers.
Discount pravachol online visa
In this part, approaches for establishing release specification for a given attribute based on course of functionality considerations are mentioned. The goal is to establish acceptance standards for a given attribute that will constantly ensure that this attribute, for instance, assay, will meet the acceptance criteria with a predictable likelihood. For attributes that increase or lower with time, the steadiness profile and the expected expiration date might need to be considered to establish an upper or lower limit at launch. Specifications 107 worth for an attribute that might be modeled utilizing a normal distribution. It must be noted that the process functionality is expounded to the specification limits. If the specification limits were tightened, a process that was succesful might turn out to be not succesful whereas if the limits had been widened, a course of that was not succesful might turn out to be able to meeting the specifications. In addition, for the process thought-about "capable" on the �3 level, it could turn into "not capable" if the method imply shifted. Thus, extra considerations would wish to be included if course of or measure ment drift was an issue. Three necessary factors are as follows: (1) the uncertainty in the estimate of the attribute of curiosity, for instance, assay, impurities; (2) the estimate of the change of the attribute with time; and (3) the estimate of the uncertainty in the change within the attribute with time. With regard to the estimate of attribute uncertainty, usually this includes the batchto batch variation in the attribute plus the related measurement uncertainty. However, for some tests, it might be possible to obtain marketing authorization with restricted information reflecting a somewhat worstcase analysis pending revision when further fullscale course of knowledge turn into out there. An further com plication is that data obtained from a number of stability research might show differ ent rates of loss in order that a worstcase estimate would want to be used. As proven in the figure, the batch and assay uncertainty is estimated from the preliminary data or process capability, the estimate of the loss is decided from the linear regression pattern line, and the uncertainty within the estimate of the loss is set from the lower onesided, 95% confidence band. Typically, data from multiple batches and packaging configurations need to be thought of since they may have completely different slopes and initial values. While there are many management charting approaches, one of many easiest is to plot the attribute with time and monitor the info development relative to historic or predetermined limits. The chart also exhibits lines giving the specification limits, an alert restrict, and an motion limit. The alert limit and the action limits are determined utilizing the usual deviation, of the method. In this case, the alert limit is set at a price of �2 and the motion restrict is about at �3. However, other limits can be established as acceptable primarily based on a risk evaluation of the stability of the method. Specifications 111 alert limit are used as early warning indicators that either the process or the ana lytical testing could also be trending uncontrolled. A failure of the motion limit usu ally entails a extra rigorous investigation with additional preventive actions taken to convey the process again into management. In addition, observe within the example that since drug merchandise are typically formulated at 100 percent of label claim, while there could also be occasional deviations, the outcomes should common 100% over time, and the failure to meet this must be investigated. This may involve periodic revalidation, trending of results, reviewing the impression of adjustments or improvements to the procedure, and reviewing any failures that could be as a end result of the analytical methodology. As of this writing, the failure to conduct significant investigations remains to be one of many major sources of regulatory audit observations. As mentioned earlier, the root cause of the suspect end result can generally be related to both labrelated or manufacturingrelated issues. In the first stage of the investigation, results are reviewed with the intent of figuring out apparent errors or assignable causes. This phase of the investigation is normally conducted using a guidelines format and documented on a preliminary investigation kind. If no obvious causes are identified, a second stage involving a more indepth lab investigation is then conducted to verify the preliminary remark that was con sidered to be aberrant. Level 2 � ManufacturingPhase Investigation the preliminary manufacturing investigation begins on the end of the labphase preliminary investigation, and the investigations proceed in parallel since if the root trigger is manufacturingrelated, the investigation would need to transfer forward, with time being of the essence to stop additional batch failures.
Buy pravachol australia
Piroxicam has extra e ects in the modulation o neutrophil unction by inhibiting collagenase, proteoglycanase, and the oxidative burst. Both inhibit cyclooxygenases but also antagonize prostanoid receptors to numerous degrees. Me enamate is used solely or main dysmenorrhea, and mecloenamate is used within the remedy o rheumatoid arthritis and osteoarthritis. Ibupro en is a relatively potent analgesic used in rheumatoid arthritis (as in the case o Ms. G, to relieve intermittent pain), osteoarthritis, ankylosing spondylitis, gout, and first dysmenorrhea. Naproxen has a protracted plasma hal -li e, is 20 instances stronger than aspirin, directly inhibits leukocyte unction, and causes less severe gastrointestinal opposed e ects than aspirin. The incidence o gastrointestinal opposed e ects is comparatively low, though headache and dizziness are requently reported. Diclo enac also reduces intracellular arachidonic acid concentrations by altering cellular atty acid transport. Diclo enac is a stronger anti-in ammatory than indomethacin and naproxen and is used broadly in the remedy o pain associated with renal stones. The most important adverse e ect o acetaminophen is hepatotoxicity, and clinicians are advised each to monitor and scale back the dose o acetaminophen together with different analgesics and to cut back the total daily dose o acetaminophen. Modif cation o acetaminophen by hepatic cytochrome P450 enzymes produces a reactive metabolite, which is normally detoxif ed by conjugation with glutathione. An overdose o acetaminophen can overwhelm glutathione stores, resulting in mobile and oxidative injury and, in extreme circumstances, to acute hepatic necrosis (see Chapter 6, Drug Toxicity). The relative inhibition o the 2 cyclooxygenase isozymes in any given tissue can additionally be a unction o drug metabolism, pharmacokinetics, and possibly enzyme polymorphisms. Various coxibs had been accredited or therapy o osteoarthritis, rheumatoid arthritis, acute pain in adults, and first dysmenorrhea. Ro ecoxib was withdrawn by the manu acturer rom the worldwide market in 2004 as a end result of o a rise in myocardial in arction and stroke with extended use; valdecoxib was then withdrawn in 2005. Currently permitted indications include osteoarthritis, rheumatoid arthritis, juvenile rheumatoid arthritis (2 years o age), ankylosing spondylitis, acute pain in adults, and first dysmenorrhea. Celecoxib also will increase the dangers o hypertension, edema, and coronary heart ailure, significantly at higher doses. Celecoxib is contraindicated within the remedy o ache related to coronary artery bypass surgical procedure. A primary consideration in prescribing analgesic therapy with a coxib is whether or not or not the patient has a concurrent want or an anti-in ammatory agent. I the patient requires primarily analgesia, then acetaminophen could su f ce, perhaps together with adjunct analgesics or adjunct therapies. In all instances, the risks o coxibs in patients at risk or ischemic coronary heart disease and cerebrovascular disease should be thought-about as half o the general risk/benef t evaluation. Prostanoid Receptor Mimetics Several purposes or prostanoid receptor agonists are listed within the Drug Summary Table on the finish o this chapter. Thromboxane Antagonists Both TxA2 receptor antagonists and thromboxane synthase inhibitors might theoretically symbolize energy ul and selective agents succesful o inhibiting platelet exercise and protecting in opposition to thrombosis and vascular disease. Thromboxane antagonists may function "super" platelet inhibitors in the management o patients with cardiovascular disease. TxA2 receptor antagonists, in distinction to aspirin, would even be expected to block the vasoconstrictive action o the isoprostanes. Compounds similar to dazoxiben and pirmagrel inhibit thromboxane synthase, and ridogrel is a TxA2 receptor antagonist. With ew opposed e ects, these medication halt joint destruction and bone erosion, decrease pain, calm swollen and tender joints, and limit general illness development in rheumatoid arthritis. Years o experience with this class o medicine has proven an elevated risk o severe in ections, including disseminated or extrapulmonary tuberculosis, invasive ungal in ections (Aspergillus and endemic ungi similar to Histoplasma), hepatitis B virus reactivation, and opportunistic in ections. Patients are routinely examined or latent tuberculosis previous to initiation o remedy and should be monitored or active tuberculosis during treatment.
Centraria (Iceland Moss). Pravachol.
- Dosing considerations for Iceland Moss.
- Are there safety concerns?
- How does Iceland Moss work?
- Dry cough, loss of appetite, common cold, bronchitis, indigestion, fevers, lung disease, kidney and bladder complaints, wound healing, irritation or swelling (inflammation) of mucous membranes in the mouth or throat, and other conditions.
- What is Iceland Moss?
- Are there any interactions with medications?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96519
Order pravachol pills in toronto
Patients taking ale acept could have an elevated risk o serious in ection and an increased risk o malignancy, primarily pores and skin most cancers. As mentioned beforehand, autoantibodies in opposition to sel -antigens are one mechanism o tissue harm and in ammation in systemic lupus erythematosus. Reducing the quantity o circulating B cells results in a subsequent decrease in autoantibody production and lowered disease activity. The major adverse e ects o abatacept are exacerbations of preexisting continual obstructive lung disease and elevated susceptibility to infection. It can additionally be accredited or systemic anaplastic large cell lymphoma a ter ailure o at least one multi-agent chemotherapy regimen. In a large medical trial, belatacept was as e ective as cyclosporine at inhibiting acute rejection in renal transplant recipients. Blockade of Cell Adhesion the recruitment and accumulation o in ammatory cells at sites o in ammation is an important factor o most autoimmune diseases; the only exceptions to this rule are autoimmune ailments which are purely humoral, such as myasthenia gravis. Drugs that inhibit cell migration to websites o in ammation may also inhibit antigen presentation and cytotoxicity, thus providing multiple potential mechanisms o therapeutic action. Natalizumab Inhibition of Costimulation Costimulation re ers to the paradigm that cells o the im- mune system usually require two signals or activation (see Chapter forty two, Principles o In ammation and the Immune System). Because induction o anergy may lead to long-term acceptance o an organ gra t or limit the extent o an autoimmune illness, inhibition o costimulation represents a viable technique or immunosuppression. Several therapeutic agents inhibit costimulation by blocking the second signal required or cell activation, and more such brokers are beneath improvement. Inhibition of Complement Activation the complement system mediates multiple innate immune responses (see Chapter 42). Recognition o oreign proteins or carbohydrates leads to sequential activation o complement proteins and eventual assembly o the membrane attack complicated, a multiprotein construction that can cause cell lysis. Eculizumab is a humanized monoclonal antibody directed in opposition to C5, a complement protein that mediates late steps in complement activation and triggers meeting o the membrane assault advanced. Abatacept complexes with costimulatory B7 molecules on the sur ace o antigen-presenting cells. By this mechanism, abatacept remedy seems to be e ective in down-regulating specif c T-cell populations. Inhibition of Immune Checkpoints As discussed above within the "Inhibition o Costimulation" section, the immune system uses costimulatory alerts to activate antigen-specif c immune responses (see additionally Chapter 42). In common, ligation o these molecules on T cells inhibits the immune response; the signaling pathways that mediate these inhibitory responses are subjects o active investigation. In medical studies, ipilimumab confirmed an total survival benef t in patients with unresectable or metastatic melanoma who had been beforehand treated with one or more anticancer therapies. Patients taking ipilimumab ought to be monitored or possible immunerelated hepatotoxicity and endocrinopathies. Glucocorticoids induce pro ound suppression o the in ammatory response and immune system however trigger many adverse e ects, most o that are because of drug e ects on cells exterior the immune system. Glucocorticoid receptor modulators are being sought that retain the anti-in ammatory e ects o glucocorticoids but have much less severe adverse e ects on metabolism and bone mineral homeostasis. The cytotoxic agent mycophenolate mo etil is very selective, each as a outcome of lymphocytes depend upon de novo purine synthesis and because mycophenolic acid pre erentially targets the inosine monophosphate dehydrogenase isoenzyme expressed in lymphocytes. Lymphocyte-signaling inhibitors-such as cyclosporine, tacrolimus, sirolimus, and everolimus, which goal intracellular sign transduction pathways necessary or T-cell activation-are also moderately selective. Many new inhibitors o intracellular signaling in lymphocytes are under investigation; inhibition o the Janus kinase amily appears notably promising. The idea o stopping immune-cell activation has been extended to the blockade o costimulation represented by abatacept and belatacept. Several antibody therapeutics and small molecules can be found that block immune-cell adhesion and homing, and more such brokers are underneath development. Armstrong serves as investigator and/or marketing consultant to AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Novartis, and Pf zer.
Purchase pravachol from india
Were the patients who were prescribed one drug older than sufferers given a comparator drug I these characteristics had been evenly balanced across the customers o the varied medication, there would unlikely be a problem. However, i not (or instance, i customers o ro ecoxib have been more more likely to be smokers than customers o celecoxib, or less prone to take prophylactic doses o aspirin), this would have to be adjusted or in the evaluation. Such adjustment may be completed by statistical techniques that embrace multiple regression, propensity scores, or instrumental variable methods. In a randomized trial, topics are assigned arbitrarily to one treatment versus another. I the examine is large enough and the randomization works adequately, di erences in outcomes between subjects in the various research arms are likely to be the outcome o the di erent treatments they acquired as a end result of they were (by def nition) related in all different respects. By contrast, in an observational study, the researcher is obliged to research outcomes in patients or whom a doctor has already chosen to prescribe one drug versus another versus no drug. It is there ore essential to move beyond the straightforward 2 2 ormulation described above, adjusting the noticed relationships in order to management or di erences that will have existed be ore the sufferers took the medication beneath research. However, the early reports o increased threat counsel that selection bias could present another explanation or suicide in uoxetine users. Thus, a doctor would pre er a potentially suicidal patient to have a provide o uoxetine at house somewhat than a provide o tricyclic antidepressant. Whatever the underlying danger o suicide caused by both drug, these actors alone would mix to create a prof le o greater suicide charges among new uoxetine users in comparability with tricyclic antidepressant users in an observational evaluation. Such sufferers are probably also more prone to interact in different health-promoting behaviors, corresponding to tobacco avoidance, weight control, train, and adherence to their other prescribed drug regimens. These traits likely exist to a good larger extent amongst patients who adhere aith ully to the prescribed routine or a chronic interval o time. The solution is not to embrace all di erences in opposed e ect rates regardless o their statistical properties. However, when the info rom all such trials had been aggregated (in some circumstances, years a ter the studies have been completed), it grew to become clear that the chance across all studies was clear and consistent (and additionally met the conventional p zero. The opposite drawback arises when contemplating the statistical signif cance o knowledge rom giant population-based epidemiologic research. But right here, even i the f nding seems to have statistical signif cance, a di erence o such small magnitude might have little or no scientific importance. As a end result, the concept o risk administration has turn out to be an important theme in drug development and regulation. However, extra refined readers o the literature understand that such a cut point is essentially arbitrary (compared to , or instance, a p value o 0. The scenario is even more crucial in assessing the statistical signif cance o information about adverse events, whether rom a randomized trial or rom an observational evaluation. It is use ul to recall that the p value is set by each sample dimension and the magnitude o an noticed di erence. In this case, nevertheless, the absence o head-to-head medical trials makes it di f cult to make such an analysis. Thus, in most cases, the person clinician is le t to make therapeutic decisions with out the info wanted to make such choices rigorously. A current improvement designed to treatment this problem is the movement toward comparative e ectiveness research-a program o publicly unded studies that systematically consider therapies in opposition to each other. Industry critics have argued that these materials o ten emphasize therapeutic benef ts more persuasively than they impart risk. As another, several innovative applications have emerged that provide prescribers with noncommercial, publicly unded "advertising" o evidence-based information about drug benef ts, risks, and prices, often known as academic detailing. Juries and courts have agreed with this notion; legal settlements exceeded $1 billion or cerivastatin (Baycol) and $21 billion or dex enf uramine (Redux), even in the absence o legal convictions. These knowledge are being made even more use ul by advanced methodological instruments, similar to propensity scores and instrumental variables, to improve control or con ounding in observational research. Pharmacoepidemiologic analyses based on these developments can orm the oundation or decisions-made each at the bedside and at coverage levels-based on science rather than on hunches, ear, or hype. These databases and epidemiologic instruments also hold potential or def ning comparative drug e ectiveness through the use of the same instruments to measure desired medical outcomes throughout brokers.
Order pravachol 10mg visa
The first three subtypes are categorised as standard danger, while the two other subtypes constitute high-risk illness. A main change within the method to this disease recently has been the introduction of molecular Table 14. Recent data counsel molecular subtyping can be utilized to complement staging to outline prognostic groups. Four primary subgroups defined by genomic analysis have been characterized at an international consensus conference in 2010. Group 3 tumours carry a particularly poor prognosis and a high threat of metastases. In this research the low-risk group will obtain radiotherapy followed by reduced intensity chemotherapy. Management of medulloblastoma has been the subject of a sequence of multinational randomized medical research, the results of which kind the premise of current recommendations. Current normal treatment is maximal debulking surgical procedure followed by radiotherapy to the whole craniospinal axis, with a boost to the primary web site, usually the posterior fossa. Studies by which craniospinal irradiation dose was decreased further in standard-risk patients without the addition of chemotherapy confirmed lower local control rates. Growth can be affected because of native effects of radiotherapy, for example on the bony skeleton as nicely as through effects on the hypothalamic�pituitary axis. These sufferers may have development hormone and should be monitored to examine for normal pubertal improvement, as they could additionally require intercourse hormone supplementation at puberty and be vulnerable to subfertility in the long run. This is likely a reflection of radiation dose to the cochlea, combined with platinum-based chemotherapy. Posterior fossa syndrome, otherwise often recognized as cerebellar mutism, can lead to extreme language, motor, emotional and behavioural sequelae. Conclusion and learning points Medulloblastoma is a standard brain tumour in children underneath 15. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated scientific and molecular evaluation. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Schache Case history A 60-year-old man presented with a sensation of a lump on the left aspect of the throat and ipsilateral otalgia. He was in any other case fit and well, a non-smoker and teetotaller, with an Eastern Cooperative Oncology Group efficiency status of zero. Clinical examination revealed a mass primarily based throughout the left tonsil and palpable lymph nodes on the left side of the neck. On the premise of scientific and radiological features, the patient was staged as having a T2N2bM0 left tonsil tumour. He underwent pan-endoscopy and biopsy beneath basic anaesthetic, which confirmed non-keratinizing, moderately differentiated squamous cell carcinoma. The tumour was macroscopically fully excised, albeit with microscopically concerned margins (<1 mm; unfavorable marginal biopsies). The affected person was stratified to the high-risk group and randomized to obtain adjuvant chemoradiotherapy (60 Gy in 30 fractions with cisplatin one hundred mg/m2 on days 1 and 22). He completed remedy as per protocol; nevertheless, the chemoradiation induced extreme (grade 3) mucositis, inflicting decreased oral consumption, weight loss and severe pain requiring admission to the hospital for over a week for symptom management. Two principal approaches may be applied: either primary surgical procedure adopted by radiotherapy/chemoradiotherapy, depending on postoperative pathology, or major chemoradiation with salvage surgery if required. In addition, radiotherapy could also be combined with cetuximab as an alternative of chemotherapy as primary remedy. There is, to date, no randomized evidence proving the prevalence of one strategy over the other, and outcomes appear to be comparable. Choice of remedy routine partly is dependent upon native experience, patient comorbidity (such as renal impairment that will restrict the use of cisplatin chemotherapy), tumour location and certain impact on practical outcomes, and, critically, affected person choice. Notably, the place radiation is the first treatment in superior illness, the addition of chemotherapy has been shown significantly to enhance survival. In addition to early toxicity, nevertheless, sufferers frequently suffer from late effects which could be permanent and impact significantly on high quality of life. Swallowing dysfunction is a key determinant of quality of life after treatment, significantly as a proportion of patients would require everlasting use of a feeding tube.
Order pravachol mastercard
Resistance to sul adoxine�pyrimethamine was reported a ter the mixture was launched in 1971 as a second-line therapy or treating people with chloroquine-resistant P. Resistance to sul adoxine� pyrimethamine was initially reported in Southeast Asia but is now relatively widespread in South America and prevalent in A rica as well. Many actors contribute to the event o drug resistance by malaria parasites, together with inappropriate and/or unsupervised drug use, inconsistent drug availability, poor adherence to remedy regimens as a result of adverse e ects and different actors, inconsistent high quality o drug manu acturing, presence o counter eit drugs, and prohibitive drug prices. Five p.c to 10% o people who stay in poverty in the developing world have serologic evidence o earlier E. Trophozoites typically multiply superf cial to the muscularis mucosae o the intestines and spread laterally. They may penetrate extra deeply, sometimes per orating the intestinal wall and spreading regionally. Inside the human physique, the trophozoites move utilizing pseudopods and ingest bacteria, other protozoa, and host pink blood cells. Symptoms as a end result of amebiasis range rom diarrhea and belly cramps to ulminant dysentery and hepatic abscess ormation. Fewer than 40% o individuals with amebic dysentery develop ever, and microscopic evaluation o the stool sometimes discloses ew neutrophils. The onset o signs can vary rom a ew days to a yr a ter publicity, or signs could never happen. Fermentation Pathways Life Cycle of Entamoeba histolytica Colonic in ection with E. Whether intestinal invasion happens may be a unction o the number o cysts ingested, the strain o the parasite, the motility o the host gastrointestinal tract, and the presence o acceptable enteric micro organism to E. Ameba additionally lack enzymes o oxidative phosphorylation, the Krebs cycle, and pyruvate dehydrogenase. Instead, ameba (and many anaerobic organisms) utilize novel enzymes to present a supply or the electron trans ers that drive metabolism. Ingestion of Entamoeba histolytica cysts can lead to a number of totally different clinical outcomes, starting from asymptomatic excretion of the cysts to invasive illness. Invasive disease outcomes when active trophozoites invade the intestinal epithelium. This invasion may end up in asymptomatic colonization, intestinal amebiasis (amebic dysentery)- which is characterized by diarrhea and abdominal cramps-or intestinal perforation. This activity is in distinction to that o heme and cytochromes, which use iron facilities to trans er electrons beneath oxidizing (aerobic) conditions. Indeed, phylogenetic analyses suggest that most o the genes encoding parasite ermentation enzymes, and many o the genes encoding parasite enzymes involved in core power metabolism, have been laterally trans erred rom anaerobic micro organism. Although lateral gene trans er is awfully requent between micro organism, it microbial cells that possess a big unfavorable redox potential; such redox potentials are present in lots of anaerobic or microaerophilic luminal parasites. However, in poorly oxygenated tissues corresponding to abscesses, metronidazole could be activated. The widespread use o metronidazole has led to drug resistance in Helicobacter pylori, a typical bacterial trigger o gastritis and peptic ulcers (see Chapter 47, Integrative Inf ammation Pharmacology: Peptic Ulcer Disease). Metronidazole resistance among luminal parasites has not yet turn out to be clinically important, however. There are three explanations or the gradual development o resistance to metronidazole among luminal parasites. Adverse e ects o metronidazole embrace gastrointestinal discom ort, complications, occasional neuropathy, a metallic style, and nausea. Metronidazole also causes nausea and f ushing when taken concomitantly with alcohol (a socalled disul ram-like e ect, attributable to inhibition o ethanol metabolism). There ore, people with invasive amebiasis are typically treated rst with metronidazole (to eradicate trophozoites which are actively invading human tissue) after which with a second agent that has more intraluminal exercise, corresponding to iodoquinol or paromomycin. The latter two agents kill ameba by unknown mechanisms however are poorly absorbed rom the gastrointestinal tract and there ore reach excessive concentrations within the lumen o the colon. Fermentation enzymes of anaerobic organisms and mechanisms of metronidazole activation. Metronidazole is a prodrug; it incorporates a nitro group that have to be decreased for the drug to turn into energetic. Two elements of anaerobic metabolism present alternatives for selective discount of the nitro group.