Allopurinol dosages: 300 mg
Allopurinol packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills
Generic allopurinol 300mg mastercard
What is the distinction between the role of the poly (A) tail in eukaryotes and in prokaryotes What are the particular nucleotide sequences and proteins needed for proper splicing of introns from the first transcript This processing consists of chopping out the intervening, non-coding sequences known as introns, and splicing together the ends to generate a shorter transcript. Addition of extra nucleotides and the modifications of current nucleotides are also necessary. After attachment of the cap, the guanine base is additional modified by addition of a methyl group. Several proteins recognize specific sequences on the 3 end of a transcript and minimize the end of the transcript off and then add numerous (100-200) adenine residues. The splicing out of the introns will be mentioned following the Focus on Relevant Research. What do you assume would occur if the introns were incorrectly spliced out of the first transcript Apoptosis occurs when the cell has accrued too many mutations or something else is inaccurate and not fixable inside the cell, corresponding to uncontrolled cell growth, most cancers, and viral infections. The splicing out of introns takes a number of steps and requires a ribonucleoprotein advanced known as the spliceosome. After addition of the 5 cap and 3 poly(A) tail, the introns are removed by splicing. Twintrons are produced when one intron is embedded inside another, creating a posh arrangement, with the splicing of the innermost intron occurring first. Alternative splicing is used in totally different cell types of the identical organism to produce completely different proteins from the identical gene. Since the cells must select which splice sites to use, the management of splicing relies upon a quantity of regulatory proteins. Cleavage of earlier tail websites might result in loss of furthermost exons, thus changing the coding sequence. Trans-splicing involves splicing collectively exons from two totally different primary transcripts. Although rare in vertebrates, trans-splicing happens reasonably usually in lower eukaryotes corresponding to nematodes and in addition within the chloroplasts of plant cells. Inteins are intervening sequences in proteins that are spliced out posttranslationally. After the splicing out of inteins, exteins are joined together to yield the final protein product. Inosine is acknowledged in translation as a guanosine, and due to this fact, probably changes the encoded protein. In an A-to-I modification, the inosine is read as a guanosine and has the potential to change the codon and amino acid at that position within the protein product. In mice, the knockout is embryonically lethal, possibly as a end result of a failure to produce stem cells and widespread apoptosis. The nuclear pores studded alongside the double membrane of the nuclear envelope in eukaryotes regulate the passage of substances into and out of the nucleus. The spliceosome is a large complex hooked up to the transcript that stops its exit from the nucleus until processing is full. Proteins are the ultimate merchandise of gene expression in most cases, and the time period proteome refers to the complete set of proteins encoded by a genome, or the whole variety of proteins present in an organism at one point in time. Translation refers to the conversion of genetic data in nucleic acid "language" to protein "language. Variations within the regular theme are B) various splicing, C) polyproteins, and D) a quantity of proteins due to the use of completely different studying frames. Overview of Protein Synthesis Each protein in each organism is made using the genetic data saved in the genome. Two relatively widespread circumstances of this are known-alternative splicing and polyproteins. In eukaryotic cells, the coding sequences of genes are sometimes interrupted by non-coding areas, the introns. This is very frequent in greater eukaryotes, specifically vertebrates (see Ch. A set of proteins generated in this manner shares a lot of their sequence and construction. Finally, there are occasional oddities, such because the generation of two proteins from the identical gene as a end result of frame-shifting (see below). To assist perceive the very complex means of translation, this chapter is divided into completely different sections. Then the process of translation is broken into 4 steps: initiation, elongation, termination, and ribosome recycling.
Cheap 300mg allopurinol amex
The concept that living cells are the structural models of life was first proposed by Schleiden and Schwann within the 1830s. Many are spherical, cylindrical, or roughly cuboidal, however many different shapes are found, such because the long-branched filaments of nerve cells. Each cell is enclosed by a cell membrane composed of proteins and phospholipids and contains a complete copy of the genome (at least at the start of its life). Living cells possess the equipment to carry out metabolic reactions and generate energy and are normally able to develop and divide. This implies that residing organisms can also only arise from pre-existing organisms. The development of specialised roles by explicit cells or entire tissues is referred to as differentiation. Some specialized cells remain useful for the life span of the individual organism, whereas others have limited life spans, sometimes lasting only some days or hours. For multicellular organisms to develop and reproduce, some cells clearly need to keep a complete copy of the genome and retain the flexibility to become another organism. In single-celled organisms, similar to micro organism or protozoa, every individual cell has a whole genome and may grow and reproduce; hence, every cell is basically the same. Each cell is surrounded by a membrane and often has a full set of genes that present it with the genetic data necessary to operate differentiation Progressive modifications within the structure and gene expression of cells belonging to a single organism that results in the formation of different sorts of cell phospholipid A hydrophobic molecule found making up cell membranes and consisting of a soluble head group and two fatty acids both linked to glycerol phosphate protein Polymer made from amino acids that does many of the work within the cell 2. Essential Properties of a Living Cell At least within the case of unicellular organisms, each cell should possess the traits of life as discussed above. Each residing cell should generate its personal power and synthesize its own macromolecules. The cell membrane, or cytoplasmic membrane, is made from a double layer of phospholipids together with proteins. Phospholipid molecules consist of a water-soluble head group, including phosphate, found on the floor of the membrane, and a lipid portion consisting of two hydrophobic chains that form the body of the membrane. The phospholipids form a hydrophobic layer that significantly retards the entry and exit of water-soluble molecules. Many of the metabolic reactions concerned within the breakdown of nutrients to launch energy are catalyzed by soluble enzymes positioned within the cytoplasm. Other energy-yielding series of reactions, such as the respiratory chain or the photosynthetic system, are located in membranes. Many cells due to this fact have a tough structural layer, the cell wall, outdoors the cell membrane. Cells differentiate into all completely different styles and sizes to present specialized functions in a multicellular organism. In this determine, pink blood cells (A) are specialised to change carbon dioxide and oxygen within the tissues of humans; fibroblasts (B) provide help to numerous organs; and neurons (C) transmit alerts from the setting to the mind to elicit a response. The phospholipid layers are oriented with their hydrophobic tails inward and their hydrophilic heads outward. Hydrophilic region Hydrophobic area Hydrophilic area Integral protein Integral protein Surface protein Some single-celled protozoa, corresponding to Paramecium, have multiple nuclei within every single cell. In addition, in certain tissues of some multicellular organisms several nuclei might share the same cytoplasm and be surrounded by only a single cytoplasmic membrane. In distinction, the larger and extra difficult cells of higher organisms (animals, fungi, vegetation, and protists) are subdivided into separate compartments and are referred to as eukaryotic cells. Cellular enzymes catalyze biosynthesis of the low molecular weight precursors to protein and nucleic acids. Prokaryotic Cells Lack a Nucleus Bacteria (singular, bacterium) are the only living cells and are categorised as prokaryotes. Like all cells, they contain all of the important chemical and structural elements needed for the sort of life they lead. Typically, each bacterial cell has a single chromosome carrying a full set of genes offering it with the genetic info necessary to operate as a residing organism. The minimum number of genes needed to enable the survival of a dwelling cell is uncertain.
Purchase allopurinol cheap
The target protein is released whereas the intein plus the chitin-binding area remain bound to the column. Selection by Phage Display Most procedures in molecular biology cope with both genetic information. Display protocols are designed to determine each the gene and its encoded protein at the same time. Here, a full-length protein or a shorter peptide is fused to a coat protein of a bacteriophage in order to be displayed on the outer floor of the virus particle. Filamentous phage M13 is a well-liked choice for phage show but different bacteriophage, similar to Lambda, T7, and T4 are additionally used. Having fewer copies of the displayed peptide on the floor of the phage avoids artifacts as a end result of simultaneous binding of multiple polypeptides. The proteins to be screened are fused to virus proteins in order that they seem on the surface floor of the virus particle. The protein is displayed on the skin of the virus particle and the corresponding gene is carried on the inside 7. First, the target gene is cloned upstream of the intein sequence and a chitinbinding domain. The bacteria are lysed and release a mixture of proteins which would possibly be handed via a chitin column. The proteins with the chitin-binding domain bind to the chitin and the remaining proteins move by way of. The fusion proteins are expressed and the intruding peptides displayed on the floor of the M13 virus particles. These areas could be giant or small, however usually a couple of amino acids are important for the two proteins to work together properly. In order to identify the peptide sequence these binding sites recognize, phage display libraries are constructed. These include a lot of modified phages displaying a library of different peptide sequences. They have been screened to discover peptides that bind to particular molecules (the "target"), similar to a particular antibody, enzyme, or cell-surface receptor. The peptide of curiosity is discovered by a range procedure referred to as biopanning. The phage show library is incubated with goal molecules which might be connected to a strong help (beads or membranes, and so forth. Several cycles of binding and amplification will enrich for the phage that carries the peptide that binds most tightly to the target. Biopanning is a process by which a mix of bacteriophage particles with different proteins displayed is screened for binding to an antibody or different particular binding protein. Here, the N-terminal portion shall be on the outside of the phage particle, whereas the C-terminus shall be on the within. Therefore, the peptide have to be fused in body on the N-terminus to be displayed on the skin of the phage. Those phage that show peptides that bind to the target protein might be retained (C), but the others are washed away. The phage that does acknowledge the binding protein can then be released, isolated, and purified. It probably incorporates a lot of the theoretically possible amino acid heptamers (of which there are 207 approximately 1. Full-length proteins can additionally be fused to phage coat proteins to produce a fulllength phage show library. In precept, a gene library from any organism might be converted into a phage show library by insertion into an appropriate phage coat protein gene. Therefore, each ends of the insert must thus be in body, and in addition, no cease codons should be current in the insert. In contrast, in T7 the C-terminal area of the coat protein is uncovered on the outside.
Purchase allopurinol 300mg
Rare Amino Acids Encoded by Stop Codons l the uncommon amino acids selenocysteine and pyrrolysine are inserted at particular stop codons. Protein Degradation e255 the genetic code contains 20 amino acids, however many other amino acids are made by modifying these 20 amino acids after proteins have been made. Instead of the stop codon studying as such, it generally encodes the rare amino acid. Several proteins throughout the human body utilize selenocysteine, one of the uncommon amino acids. These are referred to as Sec-containing proteins and are sometimes concerned in combating oxidative stress through antioxidants. When selenium is scarce within the body, the liver, kidneys, and lungs must resolve which proteins that comprise selenocysteine might proceed to be synthesized. This evaluate summarizes the analysis into the investigation of how the cell prioritizes the interpretation of Sec-containing proteins. However, utilizing your current data, what mechanism do you think could be a plausible explanation for this phenomenon The action of proteases should be rigorously managed by the cell to prevent injury to non-targeted proteins. Cells will typically compartmentalize proteases to limit their involvement with other cellular structures. Additionally, no much less than in eukaryotes, proteins are tagged with ubiquitin, which is a signal for degradation. Once tagged, the proteins are fed into the proteasome, which degrades the protein and cleaves off the ubiquitin tag. There are 20 frequent genetically encoded amino acids, every with chemically distinct facet chains. The broad selection of potential monomers makes proteins extremely versatile, with a variety of properties and capabilities. In addition there are structural proteins with no catalytic or binding activities and a wide range of regulatory proteins that management both gene expression and other mobile activities. In order to fulfill their numerous roles, proteins fold up into a variety of 3D shapes and constructions. Several ranges of folding are required to generate the final construction of proteins and these rely upon a wide range of chemical forces to maintain them together. After folding of the protein itself, different chemical teams might have to be hooked up for the protein to operate appropriately. The Structure of Proteins Reflects Four Levels of Organization During the method of translation, amino acids are linked together into a linear polypeptide chain. Each amino acid has a specific chemistry that dictates how that chain interacts and folds right into a 3D construction to perform correctly. The final shape of a protein is decided by its amino acid sequence, so proteins with similar sequences have comparable 3D conformations. As more and more protein 3D constructions are deciphered, it has emerged that some proteins have similar 3D structures even when their amino acid sequences are quite totally different. However, many hormones and growth factors, similar to insulin, do encompass comparatively brief polypeptide chains. Individual polypeptides with greater than a thousand amino acids are very uncommon and very giant proteins are most likely to encompass a number of separate polypeptide chains rather than a single lengthy chain. The buildings of organic polymers, both proteins and nucleic acids, are sometimes divided into four levels of organization: 1. Secondary construction is the folding or coiling of the original polymer chains by means of hydrogen bonding. In the case of proteins, the hydrogen bonds are between the atoms of the polypeptide spine. Tertiary construction is the additional folding that gives the ultimate 3D structure of a single polymer chain. In the case of proteins, this entails interactions between the R teams of the amino acids. The Secondary Structure of Proteins Relies on Hydrogen Bonds By definition, the secondary structure is folding that depends solely on hydrogen bonding. In proteins, hydrogen bonding occurs between the peptide teams that type the backbone of the polypeptide. The polypeptide chain must be folded around to bring two peptide groups alongside each other.
Order allopurinol online
Fatty acids are taken up as coenzyme A derivatives, not free fatty acids; therefore, the signal molecule acknowledged by FadR is a longchain acyl-CoA. In the absence of acyl-CoA, FadR represses the operons for fatty acid degradation and in addition prompts fabA, a gene concerned in fatty acid biosynthesis. Fatty acid degradation is induced and in addition the extent of expression Current opinion in structural biology of fabA decreases, so fewer fatty acids are manufactured. One is determined by which molecules match the active site(s) of the enzyme(s) of the pathway and the other by which molecules match the binding site on the regulatory protein. For induction of the lac operon by lactose, low levels of both LacY (transport protein) and LacZ (-galactosidase) proteins are essential. A small amount of lactose should be transported into the cell and be transformed by -galactosidase to allo-lactose earlier than it could bind to LacI and induce. The maltose system allows transport and metabolism of maltose and longer oligosaccharides additionally manufactured from glucose subunits. The maltose system is underneath constructive management and the MalT activator protein actually binds maltotriose, a trisaccharide consisting of three glucose residues. Some repressors are solely energetic when they bind a small signal molecule known as a co-repressor. In common, the cell ought to turn biosynthetic pathways off when their products are current within the medium or have been synthesized in sufficient quantities. Occasionally, repressors or activators bind other proteins, quite than small metabolites. For example, Mlc is a repressor that regulates glucose transport and a variety of other genes concerned within the uptake and metabolism of monosaccharides. When glucose is absent, phosphate teams accumulate on the glucose transporter or PtsG protein. When glucose enters the cell, phosphate transfers from PtsG to glucose, thus changing it to glucose-6-phosphate. Unphosphorylated PtsG binds to Mlc, which sequesters the transcription factor at the cell membrane. Most typically that is carried out by the attachment of a chemical group, often phosphate (see below). Less generally, the regulatory protein is altered chemically in another method, for instance, by oxidation or reduction. Examples of bacterial regulatory proteins which would possibly be altered by oxidation or reduction are the activators OxyR and Fnr. OxyR is transformed to its active type by hydrogen peroxide or associated oxidizing brokers that oxidize sulfhydryl groups to disulfides. It then prompts a set of genes concerned in defending bacterial cells towards oxidative damage. When adequate arginine is current, the arginine acts as a co-repressor by binding to ArgR. B) Glucose enters the cell and is transformed into glucose-6-phosphate so removing the phosphate. If the supply of glucose runs out, PtsG will be in a position to retain its phosphate and Mlc is launched. In this case, an Fe4S4 iron sulfur cluster within the N-terminal domain of Fnr is decreased under anaerobic situations. The Fnr activator then activates genes concerned in anaerobic respiration, similar to these for nitrate reductase, fumarate reductase, and formate dehydrogenase. One large class of regulatory systems that use a phosphate group is the twocomponent regulatory techniques. As the name implies, two-component regulatory systems include two proteins that cooperate to regulate gene expression. The second is a sensor kinase that senses a change in the environment and changes form.
Order 300 mg allopurinol visa
For example, the AraC protein represses the araC gene and the Mlc protein (see below) represses transcription of the mlc gene. FadR represses the genes for fatty acid breakdown, but additionally activates certain genes involved in fatty acid synthesis. The cell can incorporate pre-made fatty acids into its lipids and can even break them down for power. Usually the sensor kinase is a trans-membrane protein that senses both physical situations of some sort. Outside the cell, the sensor domain of the kinase detects an environmental change, which finally ends up in phosphorylation of the transmitter area. Under anaerobic situations, ArcB phosphorylates itself after which phosphorylates ArcA. The ArcA~P regulator then represses about 20 genes which may be only required for cardio metabolism and prompts half a dozen genes needed when oxygen is absent or very low. In Mycobacterium tuberculosis, the causative agent of tuberculosis, some virulence elements are controlled by two-component regulators. The mother cell keeps a stalk that sticks to the soil or solid surfaces in fresh or sea water. After cell division, her daughter cell, called a swarmer cell, types a flagellum and swims away to a model new location the place the cell then reattaches to the floor and converts into a stalked cell. The steps from swarmer cell to stalked cell are extremely regulated in maintaining with the cell cycle. Next, the stalked cell divides into two during M-phase, forming one other swarming daughter cell that stays in G1 until it attaches to a floor. Phosphorylated CtrA (CtrA~P) is ample in G1 swarmer cells, and silences the origin of replication, maybe by precluding the binding of DnaA protein. At this level of the cell cycle, CtrA~P acts as an activator protein that induces transcription of genes utilized in G2 and M phase. In this paper, the authors identified a potential candidate that determines whether or not or not CtrA~P can promote transcription. The paper supplies proof that this protein prevents CtrA~P from activating gene transcription throughout G1-phase. SciP is simply discovered throughout G1-phase, and if SciP is depleted during G1, then CtrA activated genes which are usually repressed during G1 become activated. In G1, SciP is abundant, and binds to CtrA~P on the promoter of CtrA-regulated genes. In this state, any newly synthesized CtrA~P is free to bind to the origin of replication, thus preventing replication. As cells enter S-phase, CtrA is dephosphorylated and degraded so that the repressor advanced of SciP and CtrA~P are removed from the promoters. This moves the cell from S into G2 and converts the newly made daughter cell into a swarmer. These two regulatory events combine to prevent transcription of CtrA-activated genes and CtrA-repressed genes. At the midpoint of S-phase, CtrA is remade and phosphorylated as earlier, however the absence of SciP allows CtrA to act as an activator protein, and CtrA-activated genes are turned on. The path of motion of the phosphate group alongside the ArcB sensor protein is shown. Two-component techniques are also involved in those rare circumstances where micro organism show differentiation between several types of cell (see Focus on Relevant Research on the previous page). Phosphorelay Systems the pathway of phosphate switch in two-component regulatory systems truly entails four protein domains. In addition to the two-component regulatory systems, other control systems use phosphorelays. The variety of proteins, the whole number of phosphate-binding domains, and their arrangement varies in several regulatory methods.
Purchase allopurinol with a visa
The lacZ structural gene encodes -galactosidase, the enzyme that degrades lactose. The lac operon is regulated by the LacI repressor protein, which is encoded by the lacI gene. The upstream area of the lac operon accommodates a recognition sequence for the repressor protein, generally known as the operator (lacO in. The promoter is regulated by binding of the repressor on the operator, lacO, and of Crp protein at the Crp web site. Note that in actuality the operator partly overlaps each the promoter and the structural gene. Lactose, which consists of glucose linked to galactose, is transformed to allo-lactose, an isomer during which the identical two sugars are linked in a unique way. This transformation is carried out by -galactosidase, which normally splits lactose, but makes a small quantity of allo-lactose as a facet reaction. It is allo-lactose that actually binds to the LacI protein and acts as an inducer. Lactose is current in the milk consumed by infants and children, but adult diets usually comprise little or no. In actuality, the lactose genes are in all probability supposed to digest glyceryl-galactoside, a compound derived from breakdown of the lipids of animal cells. Activators and Repressors Participate in Positive and Negative Regulation 507 Box sixteen. Glyceryl-galactoside is both a genuine inducer, which binds to LacI protein, and a substrate for -galactosidase, which splits it into glycerol plus galactose. In retrospect, it was both fortuitous and lucky that Jacob and Monod selected a quite anomalous gene rather than a typical one. The regulation of the lac operon is easier than that of many genes which would possibly be extra fully built-in into the central metabolism of E. The AraC regulatory protein controls the transport and metabolism of the 5-carbon sugar arabinose. For instance, the widespread Rcs system has 5 major proteins (see Focus on Relevant Research on the next page). In eukaryotic cells, particularly in multicellular organisms, there are many extremely complicated sign transmission pathways, which frequently embody a number of phosphorelays. Two-component regulatory techniques and phosphorelays are also found in cyanobacteria, a category of algae that use photosynthesis to generate energy. Cyanobacteria are present in every area of interest on the Earth and have been round for eons. In truth, plant chloroplasts are believed to be degenerate cyanobacteria that grew to become symbiotic within the eukaryotic host cells. During evolution, the photosynthetic genes had been maintained and the remaining genes for independent development were lost. The micro organism use antennae called phycobilisomes to take in the available mild from the surroundings. The genes are managed by a sensor histidine kinase, RcaE, which has a light-weight sensing pigment on its extracellular surface. Light activation of RcaE triggers autophosphorylation, and then the phosphate group is transferred to RcaF. The discovery of two-component systems in cyanobacteria suggests that this sort of gene regulation has been used all through evolution. The Rcs system is widely distributed among the many gram-negative bacteria including enteric bacteria corresponding to E. The Rcs phosphorelay system has five parts: RcsF, RcsC, RcsD, RcsB, and RcsA. The sensor, RcsF, is an outer membrane lipoprotein that triggers the cascade by autophosphorylation. The authors have identified a model new domain on the RcsD protein that interacts with RcsB. Specific versus Global Control Many micro organism can develop on a variety of sugars, such as fructose (fruit sugar), lactose (milk sugar), and maltose (from starch breakdown), as nicely as glucose. In molecular terms, this implies the genes for utilizing these different sugars are switched off when glucose is on the market.
Discount 300mg allopurinol free shipping
Methotrexate, trimethoprim, and sulfonamide antibiotics are all examples of pharmaceuticals that focus on some side of nucleotide synthesis. The -subunit (DnaQ) of the core enzyme has proofreading ability and 3�-exonuclease exercise to be positive that replication is correct from era to generation. DnaE (subunit) is involved within the phosphodiester bond formation between nucleotides. Finally, HolE (subunit) has an unknown perform but likely helps stabilize the -subunit. The clamp-loader complicated is answerable for loading and unloading the sliding clamp and is made of 7 subunits, two, and one each of, �, and. Leading and Lagging Strands e153 the authors of this associated paper examined the E. Through their investigation, the authors determined the order of clamp-loader assembly. From their results, the authors decided that, �, and are monomeric, is a tetramer (although it can exist as monomers, dimers, and trimers), and is a tetramer as nicely. The authors were solely capable of detect in all 4 oligomeric states when the ionic power of the buffer was high. This suggests that the function of � is to break bigger oligomers of both and into smaller forms. When both � and have been added with and, a pentamer similar to (/)3� was detected, which was expected. Final meeting of the clamp loader advanced requires binding of, which stabilizes the trimer of / in the presence of �. The authors concluded that solely � can provoke meeting of the clamp loader and allow and to bind. In conclusion, the position of � in final meeting is to break the / tetramers, which then permits to bind and full the central pentameric ring. Discussion points One single clamp loader is needed for meeting of the replication complex. For such an important facet of life, cell division, do you suppose that these subunits and different proteins concerned in replication are conserved across the domains of life This discontinuous synthesis leads to the generation of fragments on the lagging strand called Okazaki fragments. As beforehand discussed in a Key Concept above, the origin of replication (oriC) is the location of replication initiation. Several enzymes localize to the region, most of them concerned in the initiation of every new strand. Any misguided base inserted during replication is recognized by the mismatch restore enzymes. These enzymes acknowledge the methylated parental strand and use it as a template to remove the inaccurate base and insert the correct base on the complementary, new strand. There are different Ter sites for counterclockwise and clockwise motion of the replisome on the round, bacterial chromosome. The outermost websites doubtless function a backup in case the primary Ter websites fail to cease the replication fork from shifting ahead. Tus binding at the terminus bodily blocks helicase from transferring forward, thus stalling the replication forks. Similar to eukaryotes, prokaryotic chromosomes can undergo recombination between the two daughter chromosomes while replication is proceeding. An even variety of crossing over occasions is ideal as this leaves two distinct chromosomes. However, if an odd number of crossovers happens, the two chromosomes can turn out to be covalently linked (see textbook. As replication proceeds in each instructions across the circular chromosome, ultimately the 2 replication forks meet and the daughter chromosomes separate from each other. Each daughter chromosome remains attached to the interior of the cell membrane, in order each cell elongates, the chromosomes utterly separate. One daughter cell is a stalked cell and may start replication and cell division instantly. The different daughter cell is a swarmer cell and should differentiate into a stalked cell earlier than replication can start. Additionally, CtrA binds to the origin of replication to silence it in swarmer cells until differentiation has occurred.
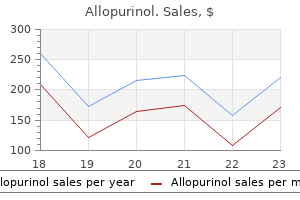
Real Experiences: Customer Reviews on Allopurinol
Ur-Gosh, 32 years: The group of Minoru Yoshida created a genetic fusion of the bromodomain gene, Brdt, and the gene for histone H4 related by a linker area for flexibility.
Baldar, 53 years: Previous studies have looked for frequent genomic mutations in quite a lot of completely different cancers together with breast, lung, colon, thyroid, and ovarian most cancers.
Brontobb, 29 years: The Xist gene of the energetic X-chromosome is inactivated by methylation, and the Xist gene on the inactivated X-chromosome is transcribed.
8 of 10 - Review by W. Jack
Votes: 58 votes
Total customer reviews: 58

