Coreg dosages: 25 mg, 12.5 mg, 6.25 mg
Coreg packs: 10 pills, 20 pills, 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills
Order coreg 25 mg without a prescription
As a part of the evolving organism, the immune system processes antigen stimuli in a deterministic trend restricted by genetics, previous antigenic experience of the host, nature of the antigen, and the conditions of its presentation. The Burden of Autoimmune Diseases the existence of autoimmune diseases in people has been recognized for 100 years. A first try and provide such a basis for the establishment of the autoimmune origin of human diseases was formulated by Witebsky et al. A timely replace for these criteria has been proposed by Rose and Bona,forty three who advised a mixture of direct proof (transfer of pathogenic antibodies or T cells), indirect evidence (reproduction of disease in experimental animals), and circumstantial evidence (clinical clues) to decide an underlying autoimmune etiology for human diseases. However, you will need to note that any particular pointers have to be tailored to particular person autoimmune issues. An example for a catalog of diagnostic standards to be evaluated in a scoring system for identification of patients with a particular autoimmune illness is the report of the International Autoimmune Hepatitis Group. More latest epidemiologic research have provided even greater estimates for the comtemporary burden of autoimmune ailments. Although clearly solely approximations, it therefore seems that autoimmune ailments are much more frequent than previously thought. The prevalence/incidence rate from every study within a illness class contributed proportionately to the imply prevalence/incidence fee based on the inhabitants measurement of that research. The proportion or weight was calculated by dividing the study inhabitants denominator by the whole of all of the research inhabitants denominators for every disease. In addition, a trend toward rising incidence rates amongst most autoimmune disease has been noticed over the past few many years. These epidemiologic research additionally allow several further, if not completely surprising, conclusions. Many autoimmune circumstances are clearly understudied, and a few of the most incessantly studied ailments exhibit comparatively low prevalence charges. The cause for the seeming imbalance between the general public health burden posed by some autoimmune issues and their attraction as objects for scientific examine stays to be elucidated but will probably embody the presence or absence of efficient therapy. Pernicious anemia, the sixth commonest autoimmune illness in the United States, could be effectively managed, and due to this fact elicits only limited epidemiologic interest. In contrast, some uncommon circumstances might pose a pronounced burden to stricken people and thus warrant continued efforts to develop simpler prophylactic and therapeutic interventions. Further, the availability of certain models for autoimmune illnesses, once more not necessarily a reflection of the epidemiologic importance of the corresponding human autoimmune illness, will have an effect on choices made by researchers charting their subject of research. Additionally, as in different areas of research or clinical medicine, the funds and assets available are the results of a quantity of components that will or could not embrace the general public health burden exerted by a particular autoimmune illness. Balancing these aspects to appropriately recognize and handle the burden of autoimmune diseases, based mostly on both the stricken individual and society at giant, is a challenge that can require our continued efforts to establish, investigate, inform, and, hopefully, enhance the therapies for many autoimmune diseases. Spectra and Continua: Organ-Specific and Systemic Autoimmune Disorders, Autoinflammatory Diseases, and the Challenges of Taxonomy A perennial method in our quest to make sense of the advanced phenomena we encounter is the establishment of dichotomies, however, fraught with shortcomings, inconsistencies, and exceptions to the rule. Given that our evolving understanding of autoimmune illnesses requires a relentless reevaluation of our concepts pertaining to etiopathogenesis and efficient therapy modalities, it would be premature to abandon such a easy and nonetheless useful classification. Rather, that porous juncture between systemic and organ-specific issues may reveal hitherto unappreciated elements of pathogenesis. On the floor, the patterns of pathology result from the distribution of anatomic niches that present a suitable surroundings to "interface" antigens and immune effectors. Leaving for the moment apart the difficulties pertaining to the identification of initiating autoantigens in plenty of human autoimmune illnesses and the difficult task to correlate markers of immunologic activity (eg, autoantibodies) with trigger or consequence of tissue destruction, a very puzzling phenomenon is the seeming organ specificity of some problems in the face of autoimmune responses that target ubiquitous antigens. Another intriguing instance is the K/BxN arthritis model in which pathogenic antibodies recognize the ever-present cytoplasmic enzyme glucose-6-phosphate isomerase. Here, the preferential involvement of the joints apparently outcomes from unique properties of the regional vasculature that allow for an antibody-mediated improve of vasopermeability and amplification of pathology by extracellular glucose-6-phosphate isomerase deposition in the articular cavities. In addition, an examination of some animal fashions used for the study of explicit organ-specific autoimmune disorders further challenges the straightforward notion of restricted pathology and should present clues about etiologic commonalities of ostensibly disparate clinical autoimmune syndromes. Perhaps most prominently, McGonagle and McDermott introduced the concept of autoinflammation outlined as "self-directed tissue inflammation, the place local components at disease-prone websites decide the activation of the innate immune system.
Purchase coreg online now
Differential splicing allows individual lg molecules to function either membrane-bound receptors for the B cell that permit antigen-specific activation or as soluble effectors, which act at a distance. In vivo, correct effector operate requires extra than just antigen-specific binding; it requires profitable neutralization of the offending antigen while avoiding probably pathogenic self-reactivity. The receptor and effector capabilities of every individual lg could be localized to a separate region or area of the molecule. Each variable (V) or constant (C) domain consists of roughly 110 to 130 amino acids, averaging 12,000 to 13,000 kD. A typical light (L) chain will thus mass approximately 25 kD, and a 3 C area Cy H chain with its hinge will mass roughly 55 kD. Each of the chains contains single amino-terminal V lg domain and one, tbree, or four carboxy-terminal C lg domains. Jsion of regions of spectacular sequence variability with areas of equally impressive sequence conservation. The V domains show the greatest molecular heterogeneity, with some regions together with non-germline-encoded variability and others exhibiting intensive germline conservation throughout 500 million years of evolution. The germline exonic derivation of the sequence is shown at the high, and the protein structure is shown at the bottom. The location of the various cysteine residues that assist hold both the individual domains and the assorted Ig subunits together are illustrated. Papain digests IgG molecules above the cysteine residues in the hinge that holds the two H chains together yielding two Fab molecules and an Fc, whereas pepsin digests below releasing an (Fab)2 fragment and two particular person Fcs (which are usually degraded to smaller peptide fragments). In their function as antibodies, Igs are launched into the circulation from the place they could visitors into the tissues and across mucosal surfaces. Soluble antibodies can additionally be pressed into service as heterologous cell surface antigen receptors by means of their attachment to membrane-bound Fc receptors. The main difference between these two types of cell surface receptors is that Igs as B-cell antigen receptors present a monoclonal receptor for every B cell, whereas antibodies certain to Fc receptors endow the cell with a polyclonal set of antigen recognition molecules. This offers greater flexibility and increases the ability of the effector cells to acknowledge antigens with a number of non-self�epitopes. It is also one situation that allows the same antibody to bind divergent antigens that share equal epitopes, a phenomenon referred to as cross-reactivity. It has been estimated that triggering of effector capabilities in answer typically requires aggregation of three or more effector domains, and thus tends to contain the binding of three or extra epitopes. For antigens encoding numerous epitopes, which is extra typical of monodisperse single-domain molecules in answer, triggering of inflammatory effector functions could require the binding of a diverse Isotypes and Idiotypes Igs can even function antigens for other Igs. The heavy-chain constant areas (green) also include the hinge (yellow) between the first two domains. The heavy- and light-chain variable areas (red and dark blue, respectively) are N terminal to the heavy- (green) and light-chain (light blue) fixed regions. Complementarity figuring out area loops within the heavy- and light-chain variable regions (yellow and white) are illustrated as well. Recognition of those isotypes first allowed grouping of Igs into recognized classes. Each class of Ig defines an individual set of C domains that corresponds to a single H chain constant region gene. Examples include the cross-reactive idiotypes found on monoclonal IgM rheumatoid factors derived from individuals with blended cryoglobulinemia, every of which can be linked to the usage of individual V gene segments. This section describes the historical past of the identification of Ig and introduces fundamental terminology. Antibodies and Antigens Aristotle and his contemporaries attributed illness to an imbalance of the 4 vital humors: the blood, the phlegm, and the yellow and black biles. Ehrlich, who was the first to describe how diphtheria toxin and antitoxin interact,19 made glancing reference to "Antik�rper" in a 1891 paper describing discrimination between two immune our bodies, or substances. He later defined that antigen is a contraction of "Antisomatogen + Immunk�rperbildner," or that which induces the production of immune our bodies (antibodies). The Fab arms of the antibody targets and probes are drawn to point out their rotational orientation as planar (oval with open center), intermediate (bone shape with or without central opening), or perpendicular ("dumbbell formed"). Different complexes illustrate the range of Fab-Fab angles made possible by segmental flexibility.
Buy coreg master card
Although every H-bond is relatively weak by itself, the mixed impact of eight hydrogen bonds results in high-affinity binding. Antibody specificity derives from the truth that the carbohydrate matches right into a binding pocket, where H-bond formation depends on precise interactions with amino acid residues which would possibly be oriented concerning the pocket. Surprisingly, most of those bonds are fashioned between sugar hydroxyls and aromatic amino acids which are neither charged nor very polar at impartial pH. The crystal structures have revealed the sources of the binding vitality that ends in affinity and specificity for this carbohydrate antigen. Once again, hydrogen bonds between hydroxyl teams of the sugars and fragrant amines (Trp and Tyr) of the protein play a dominant role in figuring out affinity and specificity of binding. This antibody binds the mannose-rich oligosaccharide facet chains that kind a protecting surface, called a glycoshield, on the envelope glycoprotein gp120. The crystal structure shows that the two terminal mannose sugars of every oligosaccharide bind end on into a deep pocket of the antibody, in a cavity-type website. Additional hydrogen bonds type between the third mannose residue and the aspect chain of Asp a hundred of the heavy chain and between the fourth mannose residue and Tyr 94 of the light chain and Tyr fifty six of the heavy chain. This arrangement allows the antibody to bind one department of an oligosaccharide and the opposite department of a nearby oligosaccharide and makes it ideally suited for cross-linking the densely clustered oligosaccharides of gp120. Immunogenicity of Polysaccharide Conjugates Capsular polysaccharides are the main target of protective antibodies against bacterial infection, and, as such, are important vaccine antigens. In younger youngsters, whose maternal antibodies wane by 6 months of age and who most need immunity to pathogens similar to Haemophilus influenzae kind b and Streptococcus pneumoniae of multiple serotypes, the T-independent response to these polysaccharides is weak, no matter chain size. To immunize children, the polysaccharides have been coupled to a protein carrier to create a brand new T-dependent antigen that gained immunogenicity from T-cell assist and boosted antibody titers with each successive dose. The same technique has produced an effective vaccine in opposition to invasive disease32 and otitis media33 caused by the most prevalent serotypes of S. Davies, private communication), these contact residues comprising the antigenic determinant could cover a major area of protein floor, as measured by x-ray crystallography of antibody�protein antigen complexes. In this case, a collection of elegant studies38�40 advised that the maximum chain length a combining website might accommodate was six to eight residues, corresponding closely to that discovered earlier for oligosaccharides,thirteen,14 as mentioned beforehand. Many of the amino acid residues exposed to solvent on the floor of a protein antigen will be hydrophilic. These are prone to interact with antibody contact residues by way of polar interactions. For occasion, an anionic glutamic acid carboxyl group could bind to a complementary cationic lysine amino group on the antibody, or vice versa, or a glutamine amide facet chain may form a hydrogen bond with the antibody. Those hydrophobic residues which are on the floor can contribute to binding to antibody for precisely the same cause. When a hydrophobic residue in a protein antigenic determinant or, similarly, in a carbohydrate determinant8 interacts with a corresponding hydrophobic residue within the antibody-combining site, the water molecules beforehand involved with each of them are excluded. A thorough evaluate of these elements of the chemistry of antigen�antibody binding is in Getzoff et al. The residues that make contact with complementary residues in the antibody-combining site are called contact residues. To make contact, in fact, these residues must be exposed on the floor of the protein, not buried within the hydrophobic core. As the complementaritydetermining residues within the hypervariable areas of antibodies have been discovered to span as a lot as 30 to forty � � 15 Mapping Epitopes: Conformation versus Sequence the opposite element that defines a protein antigenic determinant, in addition to the amino acid residues involved, is the greatest way these residues are arrayed in three dimensions. As the residues are on the surface of a protein, we are in a position to additionally think of this element as the topography of the antigenic determinant. Sela42 divided protein antigenic determinants into two categories, sequential and conformational, depending on whether or not the first sequence or the three-dimensional conformation appeared to contribute probably the most to binding. On the other hand, because the antibody-combining website has a preferred topography in the native antibody, it will appear a priori that some conformations of a selected polypeptide sequence would produce a better fit than others and subsequently could be energetically favored in binding. Thus, conformation or topography must all the time play some function within the structure of an antigenic determinant. The helices are labeled A through H from the amino terminal to the carboxyl terminal. Side chains are omitted, aside from the 2 histidine rings (F8 and E7) involved with the heme iron.
12.5 mg coreg for sale
While only one molecule (perforin) successfully delivers the granzymes into goal cells, each of the granzymes can trigger cell death. However, goal cells may be selectively proof against one or one other of the granzymes (ie, by bcl-2 overexpression or by expression of viral serpins). Requirements for a single granzyme have been proven in some cases by specific immune challenges. The granzyme B gene has additionally been deleted preserving the expression of granzymes C, D, and F intact. Because granzyme A and B are probably the most abundant granzymes in T cells, granzyme A/B doubly poor mice are extra immunodeficient than the single knockouts. The probably rationalization of these results is that the opposite "orphan" granzymes (particularly H/C, K, and M),132�134 substitute for granzyme A and B. Perforin Delivery of Cytotoxic Molecules into Target Cells When the granule membrane fuses with the killer cell membrane, the granule contents are released into the synapse. Granzymes and perforin most likely dissociate from serglycin within the immune synapse before they enter goal cells. The granzymes are delivered into the goal cell (but not the killer cell) by perforin the place they initiate at least three distinct pathways of programmed cell demise. Although perforin is important for granule-mediated cytotoxicity to deliver the granzymes into cells, the granzymes are redundant, as each granzyme can independently activate cell demise. The immune system must deal with all kinds of tumors and infections, a few of which have elaborated methods to evade apoptosis and immune destruction. Some of the granzymes may have developed to disarm particular intracellular pathogens. The interplay between granzyme B and H and adenovirus illustrates how a number of granzymes might have advanced to remove important pathogens. Granzyme H potentiates the impact of granzyme B by destroying an adenoviral granzyme B inhibitor. Perforin delivers granzymes and different effector molecules into the target cell cytosol. The precursor of human perforin is a 555 amino acid protein synthesized with a 21 amino acid chief sequence. It is unclear whether multimerization to form a pore occurs earlier than or after this conformational change. Glycosylation of at least one web site is needed for focusing on perforin to cytotoxic granules, in all probability by way of binding of the glycan to the mannose-6-phosphate receptor. This model predicts that granzymes instantly move and disperse into the goal cell cytosol. However, calcium flows into the target cell through these pores and stays elevated for a few minutes. Because intracellular calcium is low in cells with an intact cell membrane, the cell senses a calcium influx as an indication of disruption of the plasma membrane. The elevated calcium triggers a mobile membrane damage response (also known as cellular wound healing) during which intracellular vesicles move to the plasma membrane and fuse their membranes to patch holes, and any damaged membrane is quickly eliminated and internalized into endosomes. Elevated cytosolic calcium activates endosomal fusion, and granzyme- and perforin-containing endosomes fuse to type large endosomes approximately 10 times bigger than normal endosomes that have been termed gigantosomes. In the endosomal membrane, perforin varieties larger and more stable pores through which granzymes start to leak out into the cytosol. About 15 minutes after cell death has been triggered, the gigantosomes rupture, releasing any remaining cargo to the cytosol where they begin to activate programmed cell demise. When the membrane repair response is inhibited, because the cell stays leaky, goal cells die by necrosis instead of by slower, regulated, and energy-dependent programmed cell demise. Although perforin is the main molecule responsible for granzyme supply, underneath some circumstances different molecules might serve that perform. For example, bacterial and viral endosomolysins can substitute for perforin in vitro (and are extensively used as laboratory reagents for intracellular delivery162) and probably might play an identical function in vivo.
Diseases
- McDonough syndrome
- Centromeric instability immunodeficiency syndrome
- Benign familial infantile convulsions
- Horton disease
- Spastic paraparesis, infantile
- Herpes simplex encephalitis
- Polysyndactyly type 4
- Limb deficiencies distal micrognathia
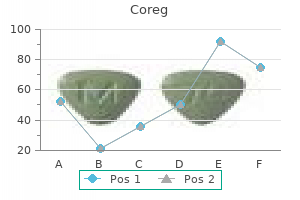
Order coreg line
Recognition of international Ig epitopes can result in sensitization and so preclude subsequent use in the identical individual of different monoclonal antibodies. Thus, monoclonal antibodies with some or all construction derived from human Ig have advantages. Several approaches have been taken using fusion of human cells with animal myelomas or with human tumor cells of assorted kinds202,203 and use of Epstein-Barr virus to immortalize antibody-producing cells. In one instance, in vitro stimulation of lymphocytes with antigen followed by fusion with mouse myeloma cells has been used to generate a series of antibodies to varicella zoster. The part of the antibody structure acknowledged as foreign by people can be minimized by combining human constant areas with mouse variable regions206,207 or even just mouse hypervariable segments208 by molecular genetic strategies. Antigen-binding specificity is retained in some cases, and the "humanized" chimeric molecules have many of some great benefits of human hybridomas. Production of totally human monoclonal antibodies in transgenic mice has now been achieved by multiple laboratories. The strategy has involved insertion into the mouse germ line of constructs containing clusters of human Ig V, D, J, and C genes (see Chapter 6) to generate one transgenic line and targeted disruption of the mouse heavy chain and chain loci to generate another transgenic line. To present feasibility of this strategy, cosmids carrying components of the human heavy chain locus had been used to make transgenic mice. Several groups utilizing different applied sciences constructed heavy chain miniloci containing practical V segments representing a quantity of main V area households, D and J segments, fixed and switch areas, and enhancers. The chain constructs were made that contained a number of functional V segments, the J segments, C, and enhancers. The human Ig genes may rearrange within the mouse genome, and expression of human Ig resulted. If these mice have been immunized with a fraction of tetanus toxin, ensuing antibodies included some that were fully human. Immunization of such mice with various antigens led to class switching, somatic mutation, and manufacturing of human antibodies of affinities of virtually 108. Ig expression in these mice demonstrates cross-species compatibility of the parts involved in antibody gene rearrangement and diversification. Oligonucleotides selected for ability to bind a ligand with high affinity and specificity are termed "aptamers" and can be used in lots of the ways antibodies have been used. These well-defined reagents could also be used increasingly in diagnostic testing and are also being tested in medical trials for use as imaging agents or therapeutics. Specificity and Cross-Reactivity Specificity of Monoclonal Antibodies Because all of the molecules in a sample of monoclonal antibody have the same variable region structure, barring variants arising after cloning, they all have the identical specificity. An exception could be an obvious cross-reaction because of a subset of denatured antibody molecules, which could possibly be eliminated on the basis of that binding. The homogeneity of monoclonal antibodies allows refinement of specificity analysis that was not potential with polyclonal sera. This capability is beneficial in designing scientific assays for related hormones, for instance. Such nice discrimination additionally allows the definition of latest specificities on advanced antigens. Another type of fine specificity analysis potential only with monoclonal antibodies is the discrimination of spatial sites (epitope clusters) by competitive binding. In some cases, such epitope clusters correspond to specificities which are readily distinguished by other means. However, in different instances, the epitope clusters will not be distinguishable by any serologic or genetic means. Only with the usage of monoclonal antibodies had been the epitopes resolved from one another. Cross-Reactions of Monoclonal Antibodies Monoclonal antibodies show many kind 1 cross-reactions, emphasizing that antibody cross-reactions symbolize actual similarities among the many antigens, not simply an effect of heterogeneity of serum antibodies. Even antigens that differ for most of their construction can share one determinant, and a monoclonal antibody recognizing this website would then give a one hundred pc cross-reaction. Antibodies to the whole vary of antigens can react with Igs in idiotype anti-idiotype reactions, displaying a cross-reactivity of the same antibodies with proteins (the anti-idiotypes) and with the carbohydrates, nucleic acids, lipids, or haptens towards which they were raised.
Generic coreg 12.5mg without a prescription
Activation by chemokines-and other leukocyte activators (eg, leukotriene-B4 or platelet-activating factor)-presented on endothelial cells causes leukocyte integrin activation, thus leading to transition from cell rolling to cell firm adhesion, in view of the power of integrin-mediated binding with endothelial immunoglobulin superfamily members. Leukocytes can then transmigrate through the endothelial monolayer and chemotactically transfer towards the inflammatory stimulus. Neutrophil-Mediated Phagocytosis Neutrophils play a important position in host safety as they get rid of microorganisms through phagocytosis (the mobile strategy of engulfi ng particles larger than 0. While many cells in our body are capable of phagocytosis, neutrophils do it to an extent sufficient to be considered "professional phagocytes" (eg, a single neutrophil can engulf up to 10 to 12 particles [eg, bacteria]). By expressing these latter receptors, neutrophils are capable of acknowledge and bind, in a cooperative manner, IgG-opsonized particles and/or complement-opsonized microbes, after which activate their phagocytosis. During the phagocytic process, the international particle is internalized, initially by way of membrane recruitment to the positioning of particle contact, and then through membrane extensions outward to surround the particle and type a model new vesicle called a cytoplasmic phagosome. The phagosome then undergoes fusion with neutrophil granules to form a phagolysosome, a protected area in which proteolytic enzymes and other bactericidal elements are discharged and pathogen degradation occurs. Remarkably, an activation of gene transcription and a selected generation of cytokines also happen during phagocytosis, a function that neutrophils utilize for enhancing a more effective innate immune response. The figure exhibits ingestion, digestion, and destruction of international particulate matter (a bacterium, in this example) by a neutrophil. A: Cell membrane receptors bind to antibody and complement molecules beforehand hooked up to the bacterial floor. C: the bacterium is trapped in a particular house, the phagocytic vacuole, into which lysosomes discharge proteases, which together with oxidants kill it. McConnell, the Nature Of Disease Pathology for the Health Professions, Philadelphia: Lippincott Williams & Wilkins, 2007. To date, a variety of stimuli in a position to induce attribute signatures of chemokine and cytokine synthesis by neutrophils have been identified. Numerous in vivo observations, in reality, not only have confirmed and reproduced the in vitro findings, however usually have clarified their organic which means and implications. The position of these molecules in mediating varied neutrophil-dependent immunoregulatory features is partially described within the following chapter. It follows that a role for neutrophils in orchestrating the sequential recruitment to , and activation of, distinct leukocyte types in the inflamed tissue is plausible, as already demonstrated to happen in a number of experimental fashions. Cytokines in daring discuss with neutrophil research in animal models that verify human findings. The latter has been clearly demonstrated in mouse fashions, during which strong Th1-dependent T-cell responses that end in pathogen clearance are elicited upon an infection with Candida albicans, Helicobacter pylori, or Legionella pneumophila. Strikingly, depletion of neutrophils reverses the Th1 responses right into a predominant Th2-response, due to this fact making the mice susceptible to an infection. More importantly, the future availability of conditional knockout mice, selectively targeting, one by one, neutrophil perform such as survival, migration, or activation, will assist to fi nally make clear the particular contributions of neutrophils beneath different inflammatory/immune settings. Role of Neutrophils in Angiogenesis and Tumor Growth There is now not doubt that, in addition to macrophages, neutrophils might positively or negatively influence the angiogenic process and tumor progress. More just lately, in vivo evidence proving that, equally to M1 and M2 macrophages, neutrophils additionally polarize from an N1, proinflammatory and antitumoral phenotype, to an N2 anti-inflammatory and protumoral phenotype, has been provided,37 thus supporting the notion that the tumor surroundings can profoundly shape the practical status of neutrophils. Dramatic clinical penalties may be noticed as a consequence of acquired neutropenia (eg, a susceptibility to infectious diseases when neutrophil counts fall under 500 cells/L4). Neutropenia can be as a outcome of depressed production (ie, hereditary neutropenias) or elevated peripheral destruction. Examples of such pathologies, tentatively categorized based on the major neutrophil-activating occasion,1 are 1) ailments attributable to ischemia reperfusion damage (ie, myocardial infarction); 2) bacterial infections (endotoxic shock, osteomyelitis, adult respiratory misery syndrome,); 3) cytokine-mediated diseases (rheumatoid arthritis, inflammatory bowel diseases); 4) illnesses brought on by crystal deposition (gout); 5) antineutrophil cytoplasmic antibody�associated vasculitis (Wegener granulomatosis, pauci-immune necrotizing crescentic glomerulonephritis); and 6) airway diseases (chronic obstructive pulmonary disease, bronchiectasis, bronchiolitis, cystic fibrosis, and even certain types of asthma are characterised by neutrophil infi ltration of the airway wall). This has been difficult to obtain, as successful remedies in animal models have incessantly confirmed ineffective or limited by unwanted effects when used in human inflammatory diseases. Herein, we focus on the specialized properties that spotlight their position within the innate and bought immune response. Eosinophils Eosinophil Generalities Eos are end-stage, multifunctional leukocytes involved in safety against parasitic helminths and bacterial and viral infections, which may be additionally implicated within the pathogenesis of quite a few Th2-type inflammatory processes and tissue harm. Eos are roughly 12 to 17 m in diameter and represent 1% to 6 % of the total blood leukocyte population. Besides circulating and massively recruited at sites of Th2-dominated irritation, Eos also reside in various organs, such because the gastrointestinal tract, mammary glands, and bone marrow. The microbicidal exercise of these cells can be induced either after direct microorganism recognition or after activation by complementor different leukocyte-derived merchandise.
Generic coreg 6.25mg without a prescription
Nucleotide sequences of all of the gamma gene loci of murine immunoglobulin heavy chains. The IgA heavy-chain gene family in rabbit: cloning and sequence evaluation of thirteen C alpha genes. Identification of a stem-loop construction essential for polyadenylation on the murine IgM secretory poly(A) website. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma reminiscence B cells. Recruitment of the cytoplasmic adaptor Grb2 to floor IgG and IgE offers antigen receptorintrinsic costimulation to class-switched B cells. Evolutionary dynamics of the human immunoglobulin kappa locus and the germline repertoire of the Vkappa genes. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambdaproducing B cells. Unraveling of the polymorphic C lambda 2-C lambda three amplification and the Ke+Oz- polymorphism in the human Ig lambda locus. Organization of the murine Igrelated lambda 5 gene transcribed selectively in pre-B lymphocytes. The murine VpreB1 and VpreB2 genes each encode a protein of the surrogate light chain and are co-expressed during B cell development. Organization of the human immunoglobulin lambda light-chain locus on chromosome 22q11. Chicken IgL gene rearrangement entails deletion of a round episome and addition of single nonrandom nucleotides to each coding segments. Characterization of coding ends in thymocytes of scid mice: implications for the mechanism of V(D)J recombination. Deletions of immunoglobulin C kappa region characterized by the round excision merchandise in mouse splenocytes. Activation of V(D)J recombination induces the formation of interlocus joints and hybrid joints in scid pre-B-cell traces. Stable expression of immunoglobulin gene V(D)J recombinase exercise by gene transfer into 3T3 fibroblasts. Similarities between initiation of V(D)J recombination and retroviral integration. Single-strand recombination signal sequence nicks in vivo: evidence for a capture model of synapsis. Coupling of V(D)J recombination to the cell cycle suppresses genomic instability and lymphoid tumorigenesis. Assembly of a 12/23 paired signal complex: a critical control level in V(D)J recombination. Ku86-deficient mice exhibit severe combined immunodeficiency and faulty processing of V(D)J recombination intermediates. Lack of N areas in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. The three enhancer region determines the B/T specificity and pro-B/pre-B specificity of immunoglobulin V kappa-J kappa joining. Increased peptide promiscuity supplies a rationale for the shortage of N regions within the neonatal T cell repertoire. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal, and hybrid joint formation. Activity of multiple light chain genes in murine myeloma cells producing a single, practical light chain. Ig heavy chain protein controls B cell improvement by regulating germ-line transcription and retargeting V(D)J recombination. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene.
Buy 12.5mg coreg mastercard
Mice ought to have a clinically relevant tumor burden or be selected for therapy when the bulk of cancer cells has been removed to undetectable ranges by surgical procedure or chemotherapy, but dormant most cancers cells stay behind to trigger later relapse. However, irrefutable evidence for the existence of tumorspecific antigens in autochthonous unmanipulated cancers solely got here after a 50-year-long search3,5,218,219,236,267�282 when in 1995, it was proven that tumor-specific antigens on cancer cells were encoded by somatic cancer-specific mutations absent within the normal cells of the host of tumor origin. Germline controls are absolutely critical for proving mutations are somatic and tumor-specific. Nevertheless, mouse tumors with proper autologous controls have additionally been used and can be found for distribution. Mice respond preferentially to nonself- or mutant� self-antigens whether brought on by genetic polymorphism or tumor-specific somatic mutation. Even when the complicated cell-cell interplay is decreased to the three molecules interacting in vitro, biochemical evaluation remains to be too complex for analyzing physiologic interactions. Proper choice is particularly essential as the quantity of peptide produced by the most cancers cell may be comparatively small and at all times must compete for binding with all different peptides naturally present within the most cancers or within the cells cross-presenting the antigen. Traditionally, "reverse immunology" has centered on finding optimal T-cell antigens, properly referred to as peptide epitopes, on infectious agents for generating vaccines. More recently, self- or mutant proteins recognized by T cells or antibodies from cancer patients have been analyzed within the seek for peptide epitopes that could be efficient targets of T-cell immunity. For instance, of the 33,345 nucleic acid base substitutions found in a human melanoma, a lot of the mutations were intergenic, intronic, noncoding, silent, or truncating. Only 187 brought on amino acid substitutions in coding genes,300 and only a few of these 187 coding substitutions are anticipated to lead to mutant peptides that function efficient targets. Only a few of the myriad of tumor-specific mutations recognized in human or murine cancers give rise to antigens that fulfill these requirements. Three questions turn into apparent: 1) Are robust antigens retained as a outcome of the host has been tolerized throughout very early stage of cancer growth Clinically, the patients who fare finest have T cells in their peripheral blood which would possibly be specific for antigens encoded by somatic tumor�specific mutations. Demonstration of the Individual (Unique) Specificity of Rejection Antigens on Independently Derived Methylcholanthrene-Induced Tumors in Transplantation Experiments. Of course, all mutations attributable to chemical and physical carcinogens are random, however virtually all of them are lost throughout cancer evolution besides people who promote the neoplastic process. When it was first shown in 1995 that cancers harbor cancer-specific antigens attributable to somatic tumor�specific mutations,10�12 each one of the three antigens represented a mutation in a tumor suppressor locus. Shared Tumor-Specific Antigens Ideally, antigens targeted on cancers would be expressed completely on malignant cells but be shared by cancers of the identical type or no much less than subtype. Several cancer-specific mutations have been recognized that are shared between cancers,9,304,326,327 but very few of them have up to now been discovered to be an effective immunologic goal. Unique tumor-specific antigens should fulfi ll the same necessities but, as a result of these distinctive mutations are extra abundant, likelihood is a lot larger to yield an effective antigen. The identical chromosomal breakpoints constantly recur in different people with the same most cancers, due to this fact end in shared tumor-specific antigens. Fusion proteins encoded by these translocations are often essential for most cancers maintenance, making them best targets for pharmacologic and immunologic intervention as a outcome of tumor cells may not easily escape remedy by losing expression of the fusion proteins. Point mutations in oncogenes can be shared by a quantity of cancers and will encode helpful antigens. One of the most effective immunologic treatments of most cancers today is the adoptive cell switch of autologous tumor-infi ltrating lymphocytes from patients with metastatic melanoma: response charges are in the range of 50% to 70% of the sufferers, and a few of these patients are cured. Similarly, medical studies show that therapeutic vaccinations with autologous most cancers cells are likely to be far more effective,319,320 confirming decades of experimental work. This is wrong in contrast with different highly individualized therapies similar to these utilized in renal transplantation. The stage of expression of those antigens can range from widespread expression to restriction to a small inhabitants or a subset of normal cells. When used as targets for destruction, T cells or antibodies should get rid of the most cancers cells while not destroying regular cells expressing the identical self-antigen, or destruction of selfantigen�expressing cells should be tolerated. Thus, no serious toxicity should happen even when the immunity is powerful enough to destroy the most cancers cells. When self-antigens are used for diagnosis, background ranges of antigen generated by regular cells complicate use of those antigens for early detection of cancer. However, adjustments within the quantity of circulating self-antigens may indicate relapse of cancer after remedy. In truth, any immune receptor binds with some affinity to any particular antigen344 and immune receptors might bind to several molecularly unrelated buildings,345 making the discussion of specificity seemingly useless.