Glucovance dosages:
Glucovance packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills

Discount glucovance 400/2.5mg with mastercard
Any discount within the overall rate of elimination of a drug as a end result of renal disease without a corresponding compensatory discount in the total rate of drug supply will result in elevated steady-state quantities within the body. This impact could, in flip, result in unwanted side effects and toxicity if the increased steady-state ranges of the drug exceed the utmost safe focus of the drug. Hence adjustment of multiple-dosage regimens by means of dose dimension, frequency of administration or each is critical if sufferers with renal disease are to avoid the potential of overmedication. Further information is offered within the specialist pharmacokinetics texts within the bibliography. Summary this article has explained the interrelationship between the rate at which a drug enters the physique and the rate at which it leaves. It has also mentioned how, in turn, this balance influences the focus of the drug in the blood plasma at any given time. It is clearly necessary for pharmacists and pharmaceutical scientists to perceive these ideas in order to find methods of sustaining therapeutic drug levels acceptable to a selected illness state. This can be achieved by the careful design of the appropriate drug delivery system. The design and formulation of modified-release drug delivery methods extending drug launch from a dosage form to delay the therapeutic drug levels within the blood plasma are discussed totally in Chapter 31. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, fourth ed. Determination of those properties for a new chemical entity is termed preformulation (literally the stage that must be undertaken before formulation proper can begin). Assay growth No related physicochemical property can be measured with out an assay, and so improvement of an appropriate assay is the primary step of preformulation. The first assay procedures should require minimal quantities of pattern (since as little as 50 mg of each compound may actually exist). For occasion, a saturated resolution ready to decide aqueous solubility may subsequently be reused to decide a partition coefficient. Initially, there could additionally be numerous potential drug candidate molecules, each with a unique set of physicochemical properties and each showing activity in direction of a particular organic target. In apply, the physicochemical properties of the molecule have an result on how a fabric will be processed pharmaceutically, its stability, its interplay with excipients and the means it will switch to answer and, finally, will determine its bioavailability. It follows that characterizing the physicochemical properties of drug candidates early within the development course of will present the elemental information base upon which candidate selection, and ultimately dosage type design, may be made, lowering development time and costs. It is an apparent level � but crucial to the task forward � that often nothing will be recognized in regards to the physicochemical properties of a brand new drug candidate, and these facts should be ascertained by a combination of scientific consideration of the molecular structure and experimentation. At this stage of the development, the new drug candidate is often considerably impure and in very brief provide. Physicochemical properties could be cut up into those which might be intrinsic to the molecule and those that are derived from bulk behaviour. No drug will attain its ultimate therapeutic target without first being in solution. It has been estimated that, traditionally, as a lot as 40% of drug candidates have been abandoned because of poor aqueous solubility, and between 35% and 40% of compounds currently in development have an aqueous solubility less than 5 mg mL-1 at pH 7. The United States Pharmacopeia and the European Pharmacopoeia present definitions of solubility based mostly on concentration (see Chapter 2 and in particular Table 2. Early dedication of solubility gives an excellent indicator as to the convenience of formulation of a drug candidate. Initial formulations, used for acquiring toxicity and bioavailability knowledge in animal fashions, will want to be liquids for oral gavage or intravenous delivery, and a solubility greater than 1 mg mL-1 is usually acceptable. For the final product, assuming oral delivery in a strong type, solubility of the molecule greater than 10 mg mL-1 is preferable. If the solubility of the drug candidate is lower than 1 mg mL-1, then salt formation, if attainable, is indicated. Dissolution is a part transition and for it to progress, solid�solid bonds should be damaged (effectively, the stable melts), while solvent�solvent bonds must be broken and replaced by solute�solvent bonds (the drug molecules turn out to be solvated) (see Chapter 2). With extra stable current, a position of equilibrium will be established between the strong and dissolved drug. The concentration of the drug dissolved at this point is recognized as the equilibrium solubility (usually referred to merely as solubility), and the answer is saturated.
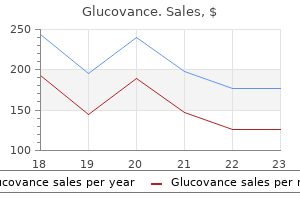
Discount 500/5mg glucovance free shipping
These dyes stay firmly adhered even after the cells have been washed with water. This sort of staining is known as simple staining, and all micro organism and other biological materials are stained the same color. Differential staining is a method more helpful process as completely different organisms and even totally different components of the identical cell can be stained distinctive colours. To put together a film prepared for staining, the glass microscope slide should be fastidiously cleaned to take away all traces of grease and dirt. If the tradition of micro organism is in liquid kind, then a loopful of suspension is transferred on to the slide. Bacteria from strong surfaces require suspension with a small drop of water on the slide to give a faintly turbid movie. When totally dry, the movie is mounted by passing the back of the slide via a small Bunsen flame until the area is just too scorching to contact on the palm of the hand. Chemical fixation is commonly carried out utilizing formalin or methyl alcohol; this causes much less damage to the specimen however tends to be used principally for blood films and tissue sections. Differential stains A large number of differential stains have been developed, and the reader is referred to the bibliography for more details. By far crucial when it comes to use and application is the Gram stain, developed by Christian Gram in 1884 and subsequently modified. Decolourization is achieved with both alcohol or acetone or mixtures of the 2. After treatment, some micro organism retain the stain and appear darkish purple and these are referred to as Gram optimistic. The colourless cells may be stained with a counterstain of contrasting colour, corresponding to 0. This methodology, although extremely helpful, should be used with caution because the Gram response may vary with the age of the cells and the strategy of the operator. For this purpose, recognized Gram-positive and Gram-negative controls ought to be stained alongside the specimen of curiosity. The bacterium responsible for the illness tuberculosis (Mycobacterium tuberculosis) accommodates within its cell wall a excessive proportion of lipids, fatty acids and alcohols, which render it resistant to regular staining procedures. The inclusion of phenol in the dye answer, along with the application of heat, enables the dye (basic fuchsin) to penetrate the cell and, once attached, to resist vigorous decolourization by robust acids. Coupling antibodies to the fluorochromes can enhance specificity, and this system has found extensive utility in microbiology. As with the staining procedures described earlier, this technique can only be applied to dead cells. The three following techniques have been developed for the examination of dwelling organisms. Dark-ground microscopy the usual operate of the microscope condenser is to focus as a lot mild as possible through the specimen and into the target lens. The dark-ground condenser performs the alternative task, producing a hole cone of sunshine that comes to a give attention to the specimen. The rays of light within the cone are at an indirect angle, such that after passing throughout the specimen, they proceed with out assembly the entrance lens of the objective, leading to a dark background. Any objects current at the point of focus scatter the light, which then enters the objective and shows up as a brilliant image towards the dark background. Specimen preparation is important, as very dilute bacterial suspensions are required, preferably with all of the objects in the identical aircraft of focus. Dust and grease also scatter light and destroy the uniformly black background required for this system. Phase-contrast microscopy this method allows us to see clear objects well contrasted from the background in clear element and is the most widely used image-enhancement methodology in microbiology. In essence, an annulus of light is produced by the condenser of the microscope and centered on the again focal airplane of the target, the place a part plate, comprising a glass disc containing an annular depression, is situated.
Generic glucovance 400/2.5mg without a prescription
Genes are often faithfully copied during the billions of cell division occasions that occur all through a lifetime. However, numerous illnesses may be traced to the mutation of assorted genes and thus have a genetic foundation. Gene mutations will thus alter the ensuing protein, and such alterations may result in disease. Disease-linked gene mutations, as in cystic fibrosis, result in a nonfunctioning protein � the nonfunctioning cystic fibrosis transmembrane regulator. Theoretically, gene therapy could additionally be used to replace a mutated gene and thus achieve a functioning protein. Proof of this therapeutic concept has been demonstrated in people through the licensed gene drugs Gendicine. Gendicine accommodates wild-type p53 to exchange mutated p53 in cancer cells and is delivered in an adenoviral vector. Delivery techniques the delivery of genes in business gene therapies has thus far been achieved with use of viruses, even though one-third of the more than 2000 gene remedy trials performed to date used artificial (nonviral) vectors or no vectors in any respect. This replicationincompetent virus performs a mutation compensation operate and is administered intratumorally. The want for intratumoral injections is a extreme limitation of the therapy as it is in all probability not easily used to treat metastatic cancers. Patients obtain one injection per week for 4�8 weeks, and each injection consists of 1012 viral particles in 1 mL of water for injections containing glycerol (to preserve tonicity). Normally p53 is upregulated in cancer cells and causes cell apoptosis, antiangiogensis, an activation of an antitumour immune response and a downregulation of the expression of the multidrug resistance genes. On intratumoral administration of Gendicine, the adenovirus enters the most cancers cell through the Coxsackie virus and adenovirus receptor and the cell then begins to overexpress wild-type p53, inflicting apoptosis. The three approved gene therapeutics (Gendicine, Glybera and Oncorine) are delivered by viral vectors. Gendicine, which is delivered in an adenovirus vector, is produced as a viral particle. The Gendicine adenovirus consists of an E1 gene deleted adenoviral vector, with E1 gene deletion essential to prevent replication. As there are some limitations related to viral vectors for gene remedy, notable amongst these being their restricted use as systemic therapeutics, a selection of research have appeared on the improvement of synthetic vectors. If artificial (chemical compound) vectors are used for gene therapy, the gene product is delivered as a bacterial plasmid. Plasmids containing the gene of curiosity are grown in Escherichia coli cell strains and purified by cell lysis, filtration, chromatographic separation and centrifugation. The drug is indicated for the remedy of lipoprotein lipase deficiency, a uncommon disease during which patients current with hyperlipidemia and pancreatitis, the latter of which is deadly. Administration of Glybera is problematic and requires intramuscular administration to 30�70 websites at any one time. Viral supply systems for gene remedy have had a turbulent history, with the high-profile demise of an otherwise wholesome patient in one adenovirus gene remedy trial, and sufferers developing leukaemia, stemming from insertional mutagenesis, in a trial involving ex vivo retroviral gene therapy for the treatment of severe mixed immunodeficiency syndrome. Good preclinical efficacy (tumour regression) data have been obtained with the poly(propylenimine) dendrimer gene remedy system. Gene silencing of the M2 subunit of ribonuclease reductase was achieved in human melanoma tissue on this pivotal human research. Summary Biopharmaceuticals (peptides, proteins, vaccines and nucleic acid medicines) are varied in chemical construction but have in frequent the presence of labile covalent bonds which are susceptible to hydrolysis both on storage or following administration. Stabilization of the useful protein entity on storage requires a fundamental understanding of the instability profile. A complete understanding of the mechanisms underpinning protein unfolding and aggregation is yet to be realized. This information gap prevents formulators from adopting a rational set of steps in the path of protein formulation. The most necessary factors to think about when one is formulating protein, peptide and gene biopharmaceuticals are the in vivo delivery challenges, i.
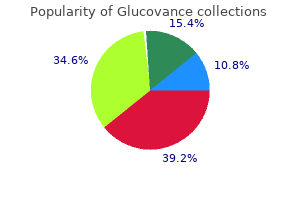
Order glucovance mastercard
It is generally accepted that the mechanism by which plasticizers exert their impact is for plasticizer molecules to interpose themselves between the polymer molecules, thus rising free quantity and facilitating increased polymer chain movement inside the structure of the coating. The optimistic advantages of this interaction embody: � elevated movie flexibility; and � lowered residual stresses within the coating because it shrinks around the core throughout drying. Some examples of commonly used plasticizers are: � polyols, similar to polyethylene glycols and propylene glycol; Whilst the number of a colourant is typically primarily based on the need to achieve a certain visual effect and, to a lesser extent, the potential affect on film mechanical properties, an underlying choice criterion is that of regulatory acceptance. Whereas there are many colourants that can be utilized, few have the complete global regulatory acceptance required to facilitate the worldwide use of the identical coating formulation. Solvents Initially, film-coating processes were very a lot depending on the use of natural solvents (such as methanol�dichloromethane combinations or acetone) in order to obtain the speedy drying traits demanded by the method. Unfortunately, natural solvents possess many disadvantages (Hogan, 1982) that are associated to the following factors: 1. Organic solvents could additionally be flammable (and thus explosive hazards) or expose plant operators to toxic hazards. Potentially unacceptable price factors associated with the use of natural solvents are associated to the necessity to construct explosion-proof processing areas and provide suitable storage areas for hazardous supplies. In addition, the relative expense of organic solvents as a raw material has to be thought of. Such disadvantages have supplied the momentum for the present utilization of aqueous coating formulations as the preferred choice. However, because of improved efficiency and decreased costs associated with solvent restoration, there was an upsurge in curiosity in solvent coating however nearly solely in modified-release coating applications. When designing film-coating formulations, formulators often discover it helpful to use specialized assessment methods that allow the properties of the person components being thought-about (including their potential useful interactions), of the coating formulations, and of the ultimate coated product to be evaluated. A evaluate of many of those helpful assessment strategies has been provided by Porter & Felton (2010). The defects which are commonly attributed to movie coating are often: Aqueous polymer dispersions Essentially, all polymers used in modified-release film-coating purposes are, to have the ability to achieve their intended functionality, usually insoluble in water. The growing demand for aqueous formulations in modern film-coating processes thus initially caused a dilemma for pharmaceutical formulators, a dilemma that has ultimately been resolved by creating aqueous polymer dispersions of many of those polymers. By far the commonest defects are these within the former category, and these have been described in detail by Rowe (1992), with recommendations for his or her resolution. These are typically associated Ideal traits of film-coated products A film-coated product and its associated manufacturing process must be designed and controlled to be certain that the following traits are evident: 588 with an imbalance between the rate of supply of the coating liquid and the rate of evaporation in the course of the drying process. This imbalance ends in both overwetting (where tablets or multiparticulates may turn into stuck together) or overdrying, when floor erosion of the tablets, in addition to chipping of the tablet edges, may outcome. These are often associated with some deficiency within the core (tablet or multiparticulate) or the coating. Coating formulation points usually lead to a movie of inadequate mechanical power, leading to film cracking and chipping, or inadequate film adhesion, resulting in movie peeling and logo bridging. Sugar coating Sugar coating has long been the normal method for coating pharmaceutical merchandise (usually tablets). It includes the successive application of sucrose-based coating formulations to tablet cores in suitable coating equipment. Conventional panning tools with guide application of syrup has been extensively used, though more specialized gear and automatic methods are actually making an impression on the method. Types of sugar coatings Sugar coatings are composed of elements that are readily soluble, or disintegrate rapidly, in water. In general, sugar-coated tablets are intended to exhibit immediate-release attributes. However, one of the phases of the sugar-coating process, the sealing step (discussed later), entails the deposition of a polymer-based coating on the floor of the uncoated tablets. As is the case with filmcoated tablets, sugar-coated tablets should be compliant with completed product specifications and any relevant compendial necessities. Traditional sugar-coating processes contain manual software methods, whereby the coating liquid 589 Ideal characteristics of sugar-coated tablets Sugar-coated tablets should possess a easy, rounded contour, with even colour protection and a glossy finish. Each of these substeps of a typical sugar-coating course of is now mentioned in turn.
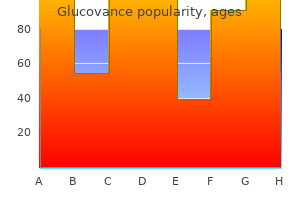
Purchase generic glucovance from india
Fenestrated endothelial linings are found within the capillaries within the kidneys and gastrointestinal tract. The basement membrane is either discontinuous, as within the capillaries within the spleen, or absent altogether, as in the case of the capillaries within the liver. Additionally, the integrity of the endothelial barrier could be disturbed by inflammatory processes or by tumour growth. This can lead to defective hypervasculature, leading to endothelial fenestrations as giant as 200 nm to 300 nm being present within the endothelial lining. In addition, because of rapid tumour progress, poor lymphatic drainage can additionally be a difficulty. Thus conjugation of a drug or protein to an acceptable soluble polymer will end in a construct with a large hydrodynamic quantity which could have reduced kidney excretion and therefore an enhanced systemic circulation time. The use of targeting groups to dictate the distribution of medicine and drug carriers can be thought-about to promote targeting to a specific site. Here, the designer of the drug delivery system is counting on the interactions between a concentrating on group, which may be covalently connected to the polymer, and a corresponding receptor to facilitate the focusing on of the system to a specific website. In this con- jugate, L-asparaginase is sure to nonbiodegradable monomethoxylpolyethylene glycol (5000 g mol-1) through an amide linker. This conjugate is used within the remedy of acute lymphoblastic leukaemia, and its mechanism of action relies on selective killing of leukaemic cells due to the depletion of plasma asparagine. This interferes with the growth of malignant cells, which, unlike most wholesome cells, are unable to synthesize L-asparagine for his or her metabolism. It can be given to sufferers with a historical past of hypersensitivity to native L-asparaginase. Antibodies and antibody-drug conjugates Antibodies are large Y-shaped protein macromolecules produced by B cells. As is the case with different nanotechnology techniques, these therapies are usually focused on oncology. In the case of antibody therapies, the antibody is designed to actively goal tumour cells. The antibody binds to the tumour cell after which acts as a marker for other elements or cells of the immune system to destroy the tumour cell. For instance, the binding of Trastuzumab acts as a marker to promote cells of the immune system to destroy the cancer cells. Fab fragments of monoclonal antibodies retain the focusing on specificity of whole monoclonal antibodies, and may even supply stronger binding; nevertheless, the fragments can be produced extra economically. Examples of antibody fragments licensed for use embody ReoPro and Lucentis (see Table forty four. There are a number of courses (or isotypes) of antibodies, with the 5 major sorts being IgG, IgA, IgM, IgE and IgD. The basic structure of IgG contains two identical heavy (50 kDa) polypeptide chains and two equivalent light (23 kDa) polypeptide chains. S Antibody conjugates Similar to polymer�drug conjugates, antibodies also can perform as carriers for medication and other brokers, together with radioisotopes and toxins. By conjugation of a molecule to an applicable monoclonal antibody, the molecules actively target the drug to the required website of action. Unfortunately, most antibody conjugates have a relatively low capacity for drugs; however, their excessive goal specificity allows them to be used effectively in the medical setting. Alternatively, the antibodies can Antibody therapies Given their ability to target a variety of specific antigens and cell sorts, antibodies can be utilized for drug concentrating on, either as medication in their very own right or as concentrating on groups for medication or delivery systems. Dendrimers Dendrimers are highly branched polymeric, starshaped macromolecules which can be prepared in the nanosize range. By various the development of the dendrimer around the core unit, one can construct dendrimers of different sizes and shapes, which might provide the power to carry drugs within the construct, or one can conjugate drugs to the floor of the dendrimer. In all cases, the resulting constructs are built to have a selected measurement, a excessive diploma of molecular uniformity and a slim molecular weight distribution. Whilst dendrimers could be considered as an evolution of branched polymers, they offer the benefit that they can be ready with a really slim measurement distribution.
Syndromes
- Rapid pulse or rapid breathing
- You should have a Pap smear ever 3 years to check for cervical cancer.
- Magnetic resonance imaging (MRI) scan
- Special blood tests to check parts of the immune system
- Healthier teeth, gums, and skin
- Very long sounds within words ("I am Booooobbbby Jones" or "Llllllllike")
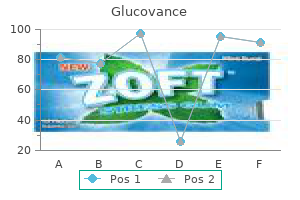
Glucovance 500/5mg amex
In order to handle a powder of acceptable flowability and bulk density, relatively large particles should be used which may be troublesome to combine to a excessive homogeneity and may be prone to segregation. Moreover, a powder consisting mainly of a drug will be troublesome to type into tablets if the drug itself has poor compactability. Finally, a uniform colouring of tablets may be troublesome to obtain with a colourant in dry particulate kind. However, granules of high quality by means of homogeneity, flowability and compactability may be ready by this operation. By spray-drying a suspension of drug particles in a liquid, which might contain a dissolved binder, relatively small spherical granules with uniform size could be ready. The course of is of limited use, except for the preparation of fillers or diluents for direct compaction. The granules can present good compactability, and this presents the potential for granulating a drug suspension without a separate drying step for the drug substance. The formation of granules by compacting the powder into massive compacts which are subsequently comminuted into smaller granules is an alternate method to granulation. The method can be used as a way of avoiding publicity of the powder to moisture and warmth and can also be referred to as dry granulation. In addition, for powders of very low bulk density, compaction may be an efficient means to increase markedly their bulk density. Depending on the meant main function, the excipients to be utilized in tablets are subcategorized into completely different teams. However, one excipient can affect the properties of a powder or the tablet in numerous methods, and plenty of substances utilized in pill formulations can thus be described as multifunctional. The features of the commonest kinds of excipients utilized in tablets are described in the following sections. Disintegrant Solution binder Dry binder Glidant Filler (or diluent) In order to type tablets of a dimension appropriate for handling, a decrease limit by way of powder quantity and weight is required. Therefore a low dose of a potent drug requires the incorporation of a substance into the formulation to increase the bulk volume of the powder and therefore the scale of the pill. The perfect filler ought to fulfil a sequence of requirements, such as: Lubricant Antiadherent � � � � be chemically inert; be nonhygroscopic; be biocompatible; possess good biopharmaceutical properties. Crystalline lactose is formed by precipitation and, depending on the crystallization situations, -monohydrate or -lactose (an anhydrous form) could be fashioned. By thermal therapy of the monohydrate form, crystalline -anhydrous particles can be prepared. Depending on the crystallization situations and the use of subsequent dimension discount by milling, lactose of various particle sizes is obtained. Amorphous lactose may be ready by the spraydrying of a lactose resolution (giving almost completely amorphous particles) or a suspension of crystalline lactose particles in a lactose answer (giving aggregates of crystalline and amorphous lactose). Amorphous lactose dissolves extra rapidly than crystalline lactose and has higher compactability. Other sugars or sugar alcohols, such as glucose, sucrose, sorbitol and mannitol, have been used as alternative fillers to lactose, primarily in lozenges or chewable tablets, due to their nice style. Mannitol has a unfavorable heat of solution and imparts a cooling sensation when sucked or chewed. Apart from the sugars, maybe probably the most extensively used fillers are powdered celluloses of different sorts. Celluloses are biocompatible, are chemically inert and have good tablet-forming and disintegrating properties. They are suitable with many drugs but, owing to their hygroscopicity, may be incompatible with medication prone to chemical degradation within the stable state. The commonest kind of cellulose powder utilized in tablet formulation is microcrystalline cellulose. The name signifies that the particles have each crystalline and amorphous areas, depending on the relative position of the cellulose chains throughout the strong. The diploma of crystallinity might differ relying on the supply of the cellulose and the preparation process. The degree of crystallinity will have an effect on the physical and technical properties of the particles. Microcrystalline cellulose is prepared by hydrolysis of cellulose adopted by spray-drying. Depending on the preparation circumstances, aggregates of various particle measurement may be prepared which have totally different flowabilities.
Buy glucovance 500/5mg on line
In turn, this could lead to a decrease within the dissolution rate and/or a lower in the rate of motion of drug molecules to the absorbing membrane. In the case of suspensions containing drugs with bioavailabilities which may be dissolution-rate dependent, an increase in viscosity might also result in a decrease in the fee of dissolution of the drug within the gastrointestinal tract. Lubricants Both tablets and capsules require lubricants in their formulation to scale back friction between the powder and steel surfaces throughout their manufacture. Magnesium stearate is often included as a lubricant during pill compaction and capsule-filling operations. Its hydrophobic nature usually retards liquid penetration into capsule components so after the shell has dissolved in the gastrointestinal fluids, a capsule-shaped plug frequently remains, especially when the contents have been formed right into a consolidated plug by a machine (see Chapter 33). Similar reductions within the dissolution rate are noticed when magnesium stearate is included in tablets. Disintegrants Disintegrants are required to break up capsules, tablets and granules into major powder particles so as to enhance the surface space of the drug uncovered to the gastrointestinal fluids. A tablet that fails to disintegrate or disintegrates slowly may result in incomplete absorption or a delay in the onset of action of the drug. Even small adjustments in formulation could end in important effects on dissolution and bioavailability. A basic example is that of tolbutamide where two formulations, the industrial product and the same formulation but containing half the amount of disintegrant, were administered to healthy volunteers. Both tablets disintegrated in vitro inside 10 minutes, Summary As well as physiological and drug elements, the dosage type can play a significant role in influencing the speed and extent of absorption. However, even with typical dosage forms, it may be very important contemplate whether or not altering the dosage kind or excipients will affect the bioavailability of the drug. Some medication will be more susceptible to changes within the price and extent of absorption through dosage form modifications than others; this can depend on the biopharmaceutical properties of the drug, which form the basis of Chapter 21. Use of pharmaceutical salts and cocrystals to handle the issue of poor solubility. Physical chemistry of supersaturated solutions and implications for oral absorption. Once the drug has been absorbed into the systemic circulation, its distribution throughout the body tissues (including to its site of action), its metabolism and its excretion are described by the pharmacokinetics of the compound (discussed in Chapter 18). This in flip influences the length and magnitude of the therapeutic impact or the response of the compound, i. As most medicine are delivered by way of the mouth, these properties will be discussed with respect to the peroral route. The bioavailability of a compound is an overall measure of its availability in the systemic circulation, and so the evaluation of bioavailability will also be mentioned. Other methods of assessing the efficiency of dosage types in vivo may even be briefly mentioned. Measurement of key biopharmaceutical properties Release of a drug from its dosage type into answer As mentioned in Chapter 20 and Part 5, a dosage form is normally formulated to aid and/or control the release of a drug from it. For example, for an immediate-release tablet, the pill needs to disintegrate to yield the primary drug particles. The solubility of a drug across the gastrointestinal pH range shall be one of many first indicators as to whether or not dissolution is liable to be fee limiting within the absorption process. Knowledge of the solubility across the gastrointestinal pH range may be decided by measuring the equilibrium solubility in appropriate buffers or by utilizing an acid or a base titration technique. Methods of measuring the dissolution fee of each a drug itself (intrinsic dissolution rate) and various 340 dosage forms are mentioned in Chapters 2 and 35, and in the chapters of Part 5. The purpose of dissolution testing is to find an in vitro attribute of a potential formulation that displays its in vivo performance. When designing a dissolution test to assess drug launch from a biopharmaceutical perspective, you will need to mimic as closely as attainable the situations of the gastrointestinal tract. Clinical scientists more and more need to rely on dissolution tests to set up in vitro�in vivo correlations between the release of the drug from the dosage kind and its absorption. Such correlations should have the benefits of reducing the use of animals to evaluate formulations and the scale and variety of pricey scientific research to assess bioavailability, as properly as getting used to enable formulation, process and website of manufacture adjustments.
Buy glucovance 500/5mg without prescription
Control of particle measurement is important as a result of particles smaller than 10 �m can move beyond the nasal turbinates towards the lung, whereas particles bigger than 50 �m could be cleared extra rapidly by mucociliary clearance and nostril blowing. An interesting advance in nasal supply gadgets which reveals useful potential in delivering nasal formulations involves breath-actuated bidirectional delivery (Table 38. The system is constructed such that the aerosolization of the powder is initiated by sufferers themselves exhaling by way of the mouth towards a resistance. This action closes the soft palate and separates the nasal cavity from the oral cavity. A communication pathway stays between the 2 nostrils, located behind the nasal septum. The expired air (and aerosolized powder) blown into one nostril is turned via 180�, passes by way of the pathway and leaves through the second nostril, guaranteeing that powder deposits throughout the cavity. Summary the potential of delivering drugs and vaccines to the body by way of the nasal cavity is far from being totally realized. There are active programmes inside a selection of pharmaceutical and biotechnological corporations looking for to use the route. It is proscribed by method of the dose that can be delivered, both on account of solubility of the drug (given the amount of liquid that can be delivered comfortably) or when it comes to the amount of powder or semisolid that can be tolerated per dose. Once drug is delivered, it should be absorbed rapidly otherwise the conventional clearance mechanisms will take away it from the absorbing epithelium and result in reduced bioavailability. Strategies are due to this fact getting used to both lengthen the absorption window (through the use of mucoadhesive excipients) or enhance permeability (by a number of means, including the usage of permeation enhancers). As with pulmonary delivery nevertheless, the first packaging is an important element of the ultimate drugs, as this also forms part of the delivery system. There is way scope to develop novel devices with improved dose dispensing and superior focusing on (perhaps to the olfactory region) or more efficient coating of mucosa. Drug development programmes need to think about each the gadget and the formulation as a whole, bearing in mind the therapeutic purpose of the medication. Nasal drug supply gadgets: characteristics and efficiency in a clinical perspective-a evaluate. The nasal 688 approach to delivering treatment for mind diseases: an anatomic, physiologic, and delivery technology overview. Intranasal supply of systemic-acting medication: small-molecules and biomacromolecules. Drug delivery to the eye is among the most important areas of contemporary ocular therapy and presents many opportunities and challenges. The current market for ophthalmic prescription drugs is value many billions of dollars a year. The entrance of the attention is accessible, and conditions affecting it can be handled by simple topical eye drops. The again of the eye, nevertheless, is treated as a wholly separate ocular area, and extra advanced delivery techniques have been designed for its remedy, including intraocular injections and implants that may provide sustained drug release over 2 years. In addition to low molecular weight molecules similar to steroids, antibiotics and antivirals, a spread of latest therapies have been and are being developed for treating ocular situations together with proteins, genes and cells. This article will describe the anatomy and physiology of the attention in addition to the commonest circumstances affecting the totally different ocular areas. The pure anatomical ocular limitations to drug bioavailability have a fantastic impact on ocular pharmacokinetics. Understanding ocular physiology is essential for creating drug supply techniques which are efficient, safe and acceptable to patients. The design of topical ophthalmic preparations starting from options to ointments and in situ forming gels shall be mentioned. The reason for these shortcomings and formulation efforts to overcome them will be described. The the rest of the chapter will concentrate on intraocular systems, together with injections and implants that deliver medicine directly to the back of the attention. This direct delivery method will increase drug bioavailability and is permitted for the delivery of several medication. Current treatment goals are to develop methods that present therapeutic drug levels throughout the eye for prolonged intervals and to minimize the invasiveness of drug delivery procedures. Intraocular implants, which have been developed by way of subtle pharmaceutical, materials and biomedical engineering approaches, are helping to achieve these targets.
Buy glucovance 400/2.5mg
One can measure the discharge of the radiolabel from the dosage type by following the depth of the radiation. By coadministration of a radiolabelled marker and a drug in the identical dosage type, and simultaneous imaging and the taking of blood samples, the absorption site and launch fee of a drug may be determined. When used in this method, the technique is usually referred to as pharmacoscintigraphy. Biopharmaceutics classification system As a result of the plethora and variability of biopharmaceutical properties of existing and potential medication, an attempt has been made to classify medicine into a small number of categories. The scheme was initially proposed for the identification of immediate-release solid oral products for which in vivo bioequivalence checks may not be needed. It can additionally be helpful to classify medicine and predict bioavailability issues that will come up in the course of the various stages of the development course of and is now used broadly by many regulatory authorities. The 4 classes are defined in phrases of excessive and low aqueous solubility and excessive and low permeability: Assessment of web site of release in vivo There are many advantages of with the ability to assess the destiny of a dosage kind in vivo, and the positioning and launch pattern of the drug. Gamma scintigraphy is now used extensively and enables greater knowledge and understanding of the transit and fate of prescription drugs within the gastrointestinal tract to be gained. The technique involves the radiolabelling of a dosage type with a -emitting isotope of applicable half-life and activity. Technetium-99m is commonly the isotope of choice for pharmaceutical research due to its quick half-life (6 h). A drug is considered to be extremely soluble when the best dose strength is soluble in 250 mL or much less of an aqueous medium over the pH vary from 1 to 8. The quantity is derived from the minimum volume anticipated in the stomach when a dosage kind is taken within the fasted state with a glass of water. The classification therefore takes into consideration the dose of the drug as nicely as its solubility. A drug is taken into account to be extremely permeable when the extent of absorption in humans is expected to be larger than 90% of the administered dose. Permeability may be assessed with one of the methods discussed earlier in this chapter that has been calibrated with recognized commonplace compounds, or by pharmacokinetic studies. Although the 2 classification methods can be used to complement one another and each have the aim of rushing, simplifying and bettering drug development, the purpose of the 2 classification methods could be very completely different. Class I medicine will dissolve quickly when offered in immediate-release dosage varieties, and are also quickly transported throughout the gut wall. This class of drug should be amenable to formulation approaches to increase the dissolution price and hence oral bioavailability. The ideas of bioequivalence and the Biopharmaceutics Classification System of drugs had been introduced. It is crucial that the biopharmaceutical properties of medication are absolutely understood, both within the choice of candidate drugs in the course of the discovery process and in the design and improvement of efficacious immediate-release and controlled-release dosage varieties. One-compartment pharmacokinetic modelling can be utilized in scientific pharmacokinetic interpretation of drug levels, providing blood sampling is finished after distribution. Most medicine show linear pharmacokinetic processes, the place the rate of elimination is proportional to the plasma focus. Some medication such as phenytoin, high-dose theophylline or salicylates and alcohol show nonlinear drug dealing with, the place elevated or a number of doses of a medicine may cause deviations from a linear pharmacokinetic profile and toxicity. The plasma�concentration time profile for a dosage form is influenced by the route of administration and the sort of formulation. At the steady state, the plasma concentration fluctuates between a maximum and a minimal stage inside a dosing interval. Changing the dose and the dosing interval will impact the extent of the fluctuations, in addition to the total concentration of the drug in the physique. Dosage regimens: affect on the plasma concentration-time profile of a drug in the physique the design of a dosage regimen determines the therapeutic profit for patients. The principles of medical pharmacokinetics are applied to design a dosage regimen for a patient that ensures the suitable formulation of the drug is chosen for an appropriate route of administration. The pharmacist needs to guarantee the appropriate regimen is prescribed to achieve optimum efficacy and minimal toxicity. Clinical pharmacokinetics supplies a fundamental understanding of the rules required to design a dosage routine.

Buy glucovance 500/5mg on-line
Surfactants in gastric juice and bile salts will have an effect on both the wettability of the drug, and therefore its efficient surface area, A, exposed to gastrointestinal fluids, and the solubility of the drug in the gastrointestinal fluids by way of micellization. The thickness of the diffusion layer, h, shall be influenced by the diploma of agitation experienced by each drug particle in the gastrointestinal tract. Hence a rise in gastric and/or intestinal motility could increase the dissolution price of a sparingly soluble drug by decreasing the thickness of the diffusion layer around every drug particle. The concentration of drug in resolution in the bulk of the gastrointestinal fluids, C, might be influenced by such elements as the rate of elimination of dissolved drug by absorption through the gastrointestinal tract and by the quantity of fluid obtainable for dissolution, which in turn might be depending on the placement of the drug within the gastrointestinal tract and the timing with respect to meal consumption. In the stomach the quantity of fluid might be influenced by the consumption of fluid within the diet. According to the Noyes�Whitney equation, a low worth of C will favour more fast dissolution of the drug by virtue of increasing the value of the time period (Cs - C). In the case of medication whose absorption is dissolution-rate restricted, the value of C is often kept very low by absorption of the drug. Hence the smaller the particle measurement, the larger the effective floor area exhibited by a given mass of drug and the higher the dissolution fee. Particle size reduction is thus more doubtless to lead to elevated bioavailability, supplied that the absorption of the drug is dissolution-rate restricted. One of the traditional examples of particle size effects on the bioavailability of poorly soluble compounds is that of griseofulvin, where a reduction of particle measurement from about 10 �m (specific surface area of zero. Many poorly soluble, slowly dissolving drugs are routinely introduced in micronized kind to improve their surface space. Examples of drugs where a discount in particle dimension has been shown to increase the rate and extent of oral absorption and hence bioavailability are shown in Table 20. For some medication, particularly these that are hydrophobic, micronization and different dry particle size discount techniques can lead to aggregation of the material. This will cause a consequent discount in the efficient floor space of the drug uncovered to the gastrointestinal fluids and hence a reduction in its dissolution rate and bioavailability. Aspirin, phenacetin and phenobarbital are all susceptible to aggregation throughout particle dimension discount. One strategy which will overcome this downside is to micronize or mill the drug with a wetting agent or hydrophilic provider. To overcome aggregation and to obtain particle sizes within the nanometre size range, moist milling within the presence of stabilizers has been used. The relative bioavailability of danazol has been increased by 400% by administering particles in the nanometre quite than the micrometre measurement range. There are now a quantity of specialised drug supply firms that can produce solid dosage forms with the drug stabilized in the nanometre dimension range to afford higher bioavailaility. Examples of commercialized merchandise are the immunosuppressant Rapamune (sirolimus), the antiemetic Emend (aprepitant) and the lipid-regulating agent TriCor (fenofibrate). It is a reformulation of the oral suspension using Nanocrystal expertise to increase the dissolution price, absorption price and bioavailability of the original formulation. The formulation is less viscous and allows 1 / 4 of the volume to be dosed, thus aiding patient swallowing and adherence. As nicely as by milling with wetting agents, the effective surface space of hydrophobic medication can be elevated by the addition of a wetting agent to the formulation. The presence of polysorbate 80 in a fine suspension of phenacetin (particle dimension less than seventy five �m) tremendously elevated the speed and extent of absorption of the phenacetin in human volunteers compared with the same-size suspension and not utilizing a wetting agent. Polysorbate eighty helps by growing the wetting and solvent penetration of the particles and by minimizing aggregation of suspended particles, thereby sustaining a large effective floor space. Wettability effects are extremely drug specific; nonetheless, wetting agents are routinely added to many formulations. For medicine similar to penicillin G and erythromycin, which are unstable in gastric fluids, their chemical degradation will be minimized if they continue to be in the solid state. Solubility in the diffusion layer, Cs the dissolution price of a drug beneath sink situations, according to the Noyes�Whitney equation (Eqn 20. In the case of medicine which are weak electrolytes, their aqueous solubility depends on pH (as mentioned in Chapter 2). Hence within the case of an orally administered strong dosage kind containing a weak electrolyte drug, the dissolution fee of the drug shall be influenced by its solubility and the pH in the diffusion layer surrounding every dissolving drug particle.