Abilify dosages: 20 mg, 15 mg, 10 mg
Abilify packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills
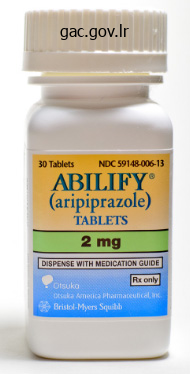
Discount 10 mg abilify with amex
The general incidence of antagonistic occasions was greater within the comparator protease arm than within the tipranavir-treated group (562. Health-related quality of life was maintained, by all measurements, in sufferers on boosted tipranavir over 48 weeks of remedy. Compared with these handled with the comparator-boosted proteases, boosted tipranavir was related to a significant however small (s. Cutaneous reactions Rash may occur with tipranavir and appears to be more widespread in ladies. The number of women in these trials was small, and maybe as a consequence the 6f. Elevated gamma-glutamyltransferase ranges had been the principle purpose for examine discontinuation, occurring in 5. No instances of scientific hepatitis or grade 3�4 triglyceride will increase were seen up to forty eight weeks. However, three sufferers developed critical bleeding occasions after 48 weeks resulting in discontinuation and one death, though these antagonistic reactions were of uncertain relationship to the examine drug. One affected person had trauma-related bruising and elevated prothrombin time, the second developed thrombotic thrombocytopenic purpura, and the third gastrointestinal bleeding as a outcome of lymphoma (Salazar et al. Subjects had not previously been treated with nonnucleoside reverse transcriptase inhibitors and had a minimal of one new nucleoside reverse transcriptase inhibitor available by genotype. They have been randomized to obtain one nonnucleoside reverse transcriptase inhibitor, one nucleoside reverse transcriptase inhibitor and either of two doses of tipranavir�ritonavir, 500/100 mg (n = 19) or 1000/100 mg (n = 22). At eighty weeks, 59% of the themes remained on remedy, 14 in the low-dose arm and 10 in the high-dose arm. Reasons for discontinuation included lack of efficacy in 5 patients, adverse occasions in 2, and serious adverse events or high-grade laboratory abnormalities in 5. Subjects had 1 or more primary protease inhibitor mutations (median 10) however not extra than 2 mutations at codons 33, 82, eighty four, or ninety. Patients had been randomized to obtain both ritonavir-boosted tipranavir or a comparator protease inhibitor, also ritonavir boosted, chosen on the basis of genotypic screening. The comparator protease inhibitors included amprenavir, indinavir, lopinavir, and saquinavir, however not darunavir. An optimized background regimen was chosen primarily based on genotype results and antiretroviral historical past. Perhaps not surprising, the greater the resistance to lopinavir (as assessed by genotypic testing), the greater the distinction in virologic response between these handled with boosted lopinavir and people given boosted tipranavir (see Table 247. Differences between remedy groups for every end result measure have been vital (p < zero. Lopinavir� ritonavir was essentially the most incessantly used comparator protease inhibitor in these trials. A subset evaluation comparing patients receiving boosted tipranavir with those receiving boosted lopinavir showed that total tipranavir was superior to lopinavir, with 39. However, no vital distinction was seen in remedy response when the tipranavir� ritonavir patients had been compared with patients receiving lopinavir�ritonavir who were naive to the drug or had virus strains totally prone to lopinavir by genotype testing. In sufferers with lopinavir susceptibility by genotype, remedy response was seen in 45. However, the conclusion was that darunavir was significantly more practical than tipranavir. A critical commentary on this examine appeared in the identical journal after the Hill and Moyle analysis was published (Llibre and Perez-Alvarez, 2007). Both drugs have been administered in combination with another selected antiretroviral medication, based on patient therapeutic history (and virtual phenotype screening results). The data from the trial, because of poor patient enrollment and untimely termination, are inadequate to assess main and secondary previously established end factors. Berhan and Berhan (2013) performed a meta-analysis of randomized controlled clinical trials in drug-experienced patients handled with tipranavir�ritonavir or darunavir� ritonavir based regimens. Tipranavir dose was 500 mg twice day by day and the twice-daily dose of ritonavir (100 or 200 mg) is proven in the table.
Buy abilify 15 mg on line
Blood screening can be utilized to monitor malathion publicity and/or toxicity in people (Liu and Pleil, 2002; Gergov et al. Any drug coming into the bloodstream is quickly degraded, even when ingested (Moeller and Rider, 1962; Baker et al. Excretion Urine is the most important route of malathion elimination in humans (Drevenkar et al. Urinalysis represents one other reliable methodology for detecting malathion toxicity and publicity (Vasilic et al. Malathion metabolites are additionally excreted in human feces, as reported in an exploratory research performed amongst 2-dayold neonates (Ostrea et al. Clinical trials assessing the efficacy or effectiveness of malathion for the treatment of head lice and scabies. Adverse reactions and toxicity 3433 Number of examine participants adopted up In every of the five groups, 50 adult fully very important lice were tested Reference Oliveira et al. The lice had been monitored at completely different points in time (5, 10, 20, 30, 60, one hundred twenty, and one hundred eighty minutes and 6 and 24 hours) Cure price at ultimate assessment Nyda L and Prioderm killed all head lice after 5 min. Treatments have been allotted semi-alternately, two sufferers given malathion to one given benzyl benzoate Hanna et al. Studies in experimental animals have additionally reported that malathion impacts cholinergic functions during embryonic growth (Aluigi et al. Studies in people have shown that chromosomal aberrations, sister chromatid exchanges, and mitotic indices have been observed in human peripheral leukocytes handled in vitro with different concentrations (0. Zeljezic and GarajVrhovac (2002) found that the mean value of sister chromatid exchange and number of cells with higher sister chromatid frequency in a population of employees occupationally exposed to a combination of insecticides together with malathion was significantly larger than in the management group. Consequently, the potential risk of chromosome injury for malathion publicity in vivo is subsequently thought of to be comparatively low. In vitro studies of the genotoxicity of malathion and its analogs, malaoxon and isomalathion, indicated that malathion is a possible mutagen and carcinogen (Blasiak et al. The reported genotoxicity of malathion may be a consequence of its metabolic biotransformation to , or the presence of, malaoxon and/or isomalathion, in addition to different unspecified impurities in commercial formulations of malathion. These findings counsel that technical grade malathion is a potent genotoxic agent and may be thought to be a possible germ cell mutagen additionally. Although direct poisonous impact of malathion on human reproductive organs has not been reported, 3434 Lindane and Malathion it has been reported that organophosphorous pesticides lower sperm high quality (Recio-Vega et al. Epidemiologic research assessing maternal publicity to individual pesticides and abortion, fetal dying, or congenital defects, while inconclusive, counsel an affiliation between the pesticides and these adverse outcomes (Garcia, 2003). Another research reported that pesticide exposure poses a danger to pregnant ladies (Goldman et al. The most specific study on the affect of malathion on pregnant women and their offspring reported that malathion metabolites could be recognized in the meconium of 2-day-old neonates (Ostrea et al. Although malathion produced teratogenic results on zebrafish embryos (Cook and Paradise, 2005; Fraysse et al. Although in a single histopathologic examine on the carcinogenic capability of malathion and its oxygen analog malaoxon in rats and mice, no carcinogenic potential was observed (Huff et al. Chemico-pathological modifications within the liver of industrial staff chronically exposed to cotton dust. Childhood aplastic anaemia in Lucknow, India: incidence, organochlorines in the blood and evaluate of case reviews following exposure to pesticides. Intestinal absorption of hexachlorobenzene and hexachlorocyclohexane and isomers in rats. Interaction between organophosphate compounds and cholinergic functions during improvement. Oxidative stress and histopathological adjustments within the heart following oral lindane (gamma hexachlorohexane) administration in rats. Concentrations of selective metabolites of organophosphorus pesticides within the United States inhabitants. In vitro research on the genotoxicity of the organophosphorus insecticide malathion and its two analogues. Comparative teratogenicity of chlorpyrifos and malathion on Xenopus laevis improvement.
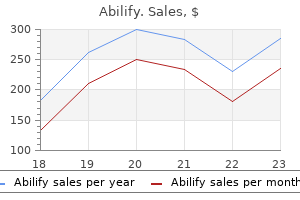
Discount 20 mg abilify mastercard
Distinct resistance patterns to etravirine and rilpivirine in viruses containing nonnucleoside reverse transcriptase inhibitor mutations at baseline. Liquid chromatography-tandem mass spectrometric assay for the non-nucleoside reverse transcriptase inhibitor rilpivirine in human plasma. Pharmacokinetics, safety and transplacental passage of rilpivirine in pregnancy: two circumstances. Lack of an effect of rilpivirine on the pharmacokinetics of ethinylestradiol and norethindrone in healthy volunteers. Clinical perspective on drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor rilpivirine. Pharmacokinetic parameters of once-daily rilpivirine following administration of efavirenz in healthy subjects. Plasma tenofovir, emtricitabine, and rilpivirine and intracellular tenofovir diphosphate and emtricitabine triphosphate pharmacokinetics following drug intake cessation. Quantification of rilpivirine in human plasma, cervicovaginal fluid, rectal fluid and genital/rectal mucosal tissues utilizing liquid chromatography-tandem mass spectrometry. A liquid chromatographytandem mass spectrometry assay for quantification of rilpivirine and dolutegravir in human plasma. A randomized, controlled trial of the effect of rilpivirine versus efavirenz on cardiovascular danger in healthy volunteers. A randomized, potential study of phenotype susceptibility testing versus standard of care to manage antiretroviral remedy. Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. Efficacy and security 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. Rilpivirine publicity in plasma and sanctuary site compartments after switching from nevirapine-containing mixed antiretroviral therapy. Skeletal muscle toxicity associated with emtricitabine/rilpivirine/tenofovir fixed-dose mixture: a case report. Agonism of human pregnane X receptor by rilpivirine and etravirine: comparison with first technology nonnucleoside reverse transcriptase inhibitors. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in wholesome volunteers. Gastrointestinal pH measurement in rats: influence of the microbial flora, food regimen and fasting. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and performance of drug transporters and drug-metabolising enzymes in vitro. Formerly known as Ro31-8959, saquinavir, whose generic name is saquinavir mesylate, was developed by Roche and is marketed underneath the trade name Invirase. Saquinavir is a peptide-based asymmetric hydroxyethylene mimetic of the transition state that occurs throughout peptide bond cleavage by aspartic proteases. The chemical name for saquinavir is cis-N-tert-butyl-decahydro-2[2(R)-hydroxy-4-phenyl-3(S)[[N-(2-quinolylcarbonyl)-L-asparginyl] amino]butyl]-(4aS, 8aS)-isoquino-line-3(S)-carboxyamide methanesulfonate. Soft gel capsules had been beforehand obtainable (marketed underneath the trade name Fortovase). Saquinavir is now rarely used in Australia, Europe, and North America, largely because of the price, tablet burden, and requirement for 200 mg/day of ritonavir co-administration. Emerging resistance and cross-resistance Resistance to saquinavir arises comparatively slowly and to a modest degree in contrast with resistance to reverse transcriptase inhibitors, such as lamivudine and nevirapine, which develops quickly. The preliminary mutation happens at place 48 (GlyVal) and subsequently at place ninety (Leu Met) and/or fifty four (IleVal) (Turriziani et al. The individual mutations at place forty eight or 90, and mutations at both of those sites, leads to successively much less processing of Gag and Gag�Pol polyproteins in vitro. The mutation at place forty eight occurs at the hinge of the beta ribbon strands close to the energetic website of the protease, doubtlessly sterically hindering entry of the inhibitor to the active website (Eberle et al. Mutations conferring resistance have been shown to arise in 45% of sufferers treated with saquinavir (1800 mg/day) alone or together with zidovudine for 8�12 months. The most common mutation observed was LeuMet at codon 90 (L90M), with mutations at codon forty eight rarely observed (Jacobsen et al. Mutations that are selected for by saquinavir present crossresistance to lots of the first-generation protease inhibitors (Winters et al.
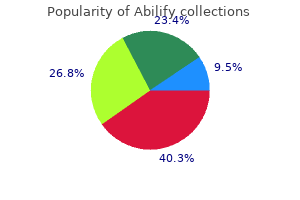
20 mg abilify with mastercard
Dose discount beneficial in gentle hepatic impairment; the product is contraindicated for reasonable to severe liver disease. In view of this, studies have been performed to examine the medical efficacy and safety of once- and twice-daily abacavir in combination with other antiretroviral medication. These studies concluded that the once-daily routine was not inferior in efficacy to the twice-daily dosing regimen (Moyle et al. However, the one double-blind, randomized managed trial that has been conducted revealed that there was a major increase within the risk of extreme drug hypersensitivity and extreme diarrhea in the arm receiving the once-daily regimen (Moyle et al. To additional cut back tablet burden, abacavir sulfate 600 mg has been co-formulated with lamivudine 300 mg (Epzicom and Kivexa), and with lamivudine 300 mg and dolutegravir 50 mg (Triumeq) for once-daily administration. Pregnant and lactating moms the pharmacokinetics of abacavir has been reported to be unchanged in pregnant women taking the drug at a dosage of 300 mg twice every day (Mirochnick and Capparelli, 2004; Best et al. Furthermore, lamivudine, which together with abacavir has potent antiretroviral exercise (Saez-Llorens et al. Abacavir is run at a dose of 8 mg/kg twice day by day in youngsters aged 3 months to sixteen years, with doses not exceeding 600 mg/day (ViiV, 2015). In a study evaluating abacavir kinetics (administered at dosage ranges of 300�600 mg) in five sufferers with various degrees of kidney dysfunction (creatinine clearance 60, forty, 25, 20 ml/minute and one patient undergoing hemodialysis), the absorption, elimination, and distribution of 5. Pharmacokinetics and pharmacodynamics 3779 abacavir had been unchanged in contrast with sufferers with normal renal operate, whereas a 4-hour hemodialysis session with a high-permeability membrane removed 24% of the drug. This examine recommended that no dosage modifications are needed for abacavir in sufferers with renal impairment and that the drug must be administered after hemodialysis to decrease drug loss (Izzedine et al. Similar recommendations have been made elsewhere (Foster and Faulds, 1998; Thompson et al. There are presently restricted knowledge on the appropriate abacavir dosage modifications in liver impairment. Abacavir clearance was decreased by 47% in the cirrhosis group, whereas the half-life of each metabolites was extended. This examine suggested that a dose of abacavir a hundred and fifty mg twice day by day be really helpful in patients with mild hepatic impairment (Raffi et al. Bioavailability the excessive water solubility (> 80 mM at 25�C) and lipophilicity (1-octanol�0. The reported oral bioavailability for 300-mg abacavir hemisulfate tablets after a single dose was 83% (range 63�110%), and was comparable to abacavir oral resolution (Chittick et al. However, the intracellular half-life of the drug is significantly longer, with the half-life of carbovir triphosphate in adults reported to vary between 12 and 18 hours after once-daily administrations of 600 mg abacavir together with other antiretrovirals (Harris et al. There was a 26% discount in abacavir maximum concentration (Cmax) and a protracted rate of absorption from zero. Food additionally has no significant impact on the extent of absorption and plasma levels of abacavir oral answer (Chittick et al. Concomitant alcohol and abacavir ingestion has been shown to prolong the plasma half-life of abacavir as a result of ethanol and abacavir share a standard metabolic pathway (both are substrates of metabolism through the glucuronidation pathway). Alcohol, which can also be metabolized by alcohol dehydrogenase, shares a metabolic pathway with the abacavir mother or father compound (Zakhari, 2006). The urinary excretion of the carboxylate metabolite was decreased by 17% (McDowell et al. In another research, the co-administration of abacavir and ethanol additionally lowered the formation of the carboxylate metabolites by 75% (Ravitch et al. The security 3780 Abacavir data for this high dose (up to 24 weeks) have been established in dose-ranging scientific trials in which a development towards increased nausea and fatigue was reported with the administration of abacavir at doses greater than 600 mg/day (Staszewski et al. No other medicine have thus far been discovered to considerably influence the absorption of abacavir (de Maat et al. Combined formulations of abacavir�lamivudine and abacavir-lamivudine�zidovudine have been shown to be bioequivalent to the person medicine administered individually, in both fed and fasting states (Yuen et al. Drug distribution the key pharmacokinetic parameters of abacavir in adults and kids are summarized in Table 230. Abacavir is rapidly absorbed after oral administration, attaining peak plasma concentrations between 1. The apparent central volume of distribution after administration of a 300-mg abacavir pill was reported to range between seventy five and 1161 (Chittick et al. In a inhabitants pharmacokinetic study evaluating twicedaily doses of abacavir tablets at a hundred, 300, and 600 mg, pharmacokinetic parameters were discovered to be dose proportional across the range of doses evaluated. There had been additionally no important differences in the abacavir pharmacokinetic parameters when evaluated together with different antiretroviral drugs (zidovudine and lamivudine), and no important pharmacokinetic variations with age, gender, or body weight (Weller et al.

Buy abilify 10mg
Placebo-controlled trial to evaluate zidovudine in therapy of human immunodeficiency virus infection in asymptomatic patients with hemophilia. Zidovudine myopathy: a particular dysfunction associated with mitochondrial dysfunction. Potential use of human stem cell issue as adjunctive therapy for human immunodeficiency virus-related cytopenias. Combined therapy with recombinant granulocyte colony-stimulating factor and erythropoietin decreases hematologic toxicity from zidovudine. Quantitation of zidovudineresistant human immunodeficiency virus sort 1 within the blood of handled and untreated patients. Pharmacokinetics of zidovudine and lamivudine in neonates following coadministration of oral doses every 12 hours. Long-term security and efficacy of zidovudine in patients with advanced human immunodeficiency virus illness. Intravenous and oral zidovudine pharmacokinetics and coagulation results in asymptomatic human immunodeficiency virus-infected hemophilia patients. Frequency of multinucleoside analogueresistant genotypes observed during antiretroviral remedy. Combination treatment with azidothymidine and granulocyte colony-stimulating consider children with human immunodeficiency virus an infection. Correlation of scientific development in human immunodeficiency virus-infected youngsters with in vitro zidovudine resistance measured by a direct quantitative peripheral blood lymphocyte assay. Contribution of nucleosideanalogue reverse transcriptase inhibitor remedy to lipoatrophy from the inhabitants to the cellular degree. The pharmacokinetics and safety of zidovudine in the third trimester of being pregnant for ladies infected with human immunodeficiency virus and their infants: section I acquired immunodeficiency syndrome clinical trials group research (protocol 082). Development and significance of zidovudine resistance in children infected with human immunodeficiency virus. Pharmacokinetics of zidovudine in end-stage renal disease: affect of haemodialysis. Effects of 3-azidothymidine on platelet counts, indium-111-labelled platelet kinetics, and antiplatelet antibodies. Mechanism and site dependency of intestinal mucosal transport and metabolism of thymidine analogues. Azidothymidine is efficient in opposition to human multiple myeloma: a brand new use for an old drug Effects of bone marrow stimulatory cytokines on human immunodeficiency virus replication and the antiviral exercise of dideoxynucleosides in cultures of monocyte/ macrophages. Different sample of activity of inhibitors of the human immunodeficiency virus in lymphocytes and monocyte/macrophages. Dideoxycytidine alone and in an alternating schedule with zidovudine in kids with symptomatic human immunodeficiency virus infection. The impact of antiviral remedy on the natural historical past of human immunodeficiency virus infection in a cohort of hemophiliacs. Effect of anticancer drugs on the glucuronidation of 3-azido-3-deoxythymidine in human liver microsomes. Rapid phenotypic reversion of zidovudine-resistant feline immunodeficiency virus without loss of drug-resistant reverse transcriptase. Effect of stage of illness and drug dose on zidovudine susceptibilities of isolates of human immunodeficiency virus. Enzymatic assay for measurement of zidovudine triphosphate in peripheral blood mononuclear cells. Biological comparability of wild-type and zidovudine-resistant isolates of human immunodeficiency virus kind 1 from the same subjects: susceptibility and resistance to other drugs. Comparative results of antifungal brokers on zidovudine glucuronidation by human liver microsomes. Severe transient neonatal lactic acidosis during prophylactic zidovudine treatment. Combination therapy with zidovudine and didanosine selects for drug-resistant human immunodeficiency virus type 1 strains with distinctive patterns of pol gene mutations.
Purchase generic abilify
Peak plasma concentrations occur within 1�2 hours of oral administration and enhance in a dose-related manner (Kaul et al. Plasma levels of stavudine decline to < 10% of Cmax between 5 and seven hours after administration. The Cmax of stavudine is roughly 2-fold decrease when taken after a high-fat meal, and the time to Cmax (tmax) is extended by roughly 2. Radioimmunoassays have been used to accurately measure stavudine ranges in human topics throughout a wide therapeutic range (Zhou et al. Serum levels of stavudine may additionally be measured by quite lots of other methods, similar to reversed-phase high-performance liquid chromatography (Burger et al. There is considerable patient to patient variation in plasma concentrations Table 229. There is equal distribution of stavudine between plasma and erythrocytes (Bristol-Myers Squibb, 2012). The central nervous system penetration of stavudine in mice is low, although a single oral dose of 25 mg/kg resulted in levels of stavudine in the brain of larger than 0. In humans, studies recommend that stavudine penetrates the cerebrospinal fluid to approximately the same extent as zidovudine (see Chapter 225, Zidovudine). However, a research taking a look at sufferers, most of whom had neurological illness, on mixture remedy found only 20% stavudine levels when measured in opposition to plasma (Antinori et al. Ex vivo maternal�fetal placental switch studies recommend that stavudine crosses the placenta by simple perfusion, quickly passing from the maternal to fetal circulation. There is a linear relationship between the imply focus of the drug in the fetal and maternal circulations (Bawdon et al. Stavudine is a low-molecular-weight compound and could be anticipated to be current in breast milk (Briggs et al. This approximate ratio was confirmed in a subsequent study of fifty two women on combination therapy, with no stavudine detected in the toddler (Fogel et al. Another research confirmed an equal ratio between stavudine breast milk and plasma in ladies treated with stavudine, lamivudine, and nevirapine, once more with no transmission of stavudine to the breastfed infant (Palombi et al. In a small examine, seminal ranges of stavudine in sufferers on mixture therapy were discovered to be comparable or larger than that of plasma (Taylor et al. Clinically essential pharmacokinetic and pharmacodynamic features There are few information particularly correlating the pharmacokinetic and pharmacodynamic features of stavudine with its scientific efficacy. The virologic efficacy of stavudine is dependent upon the trough concentration of the triphosphorylated type, stavudine triphosphate, throughout the cell. The frequency and severity of mitochondrial toxicity attributed to stavudine is decided by its dose and peak plasma focus (Domingo et al. Although additionally seen with zidovudine, zalcitabine, and didanosine, stavudine has been noticed particularly regularly to exhibit clinical toxicity as a end result of its profound effects on mitochondria. Alternative or additional mechanisms can also contribute, notably altered expression of varied metabolic genes (Mallon et al. While in depth studies have documented stavudine toxicity, the large move away from use of the drug in each creating and developed world conditions, has meant that analysis has been displaced away from predictors or markers of toxicity, with the widespread availability of safer alternatives. Monitoring of surrogate markers for stavudine toxicity, significantly lactate ranges, has been of some curiosity (Lonergan et al. Although hyperlactatemia is a extreme and recognized complication of stavudine therapy, mild to moderate increases in serum lactate levels have been shown to not be predictive of subsequent severe lactic acidosis (John et al. Renal clearance of stavudine is by each active tubular secretion and glomerular filtration. In vitro experiments using isolated hepatocytes to assess the metabolic fate of stavudine demonstrated that stavudine is quickly cleaved to thymine, which is subsequently transformed to beta-aminoisobutyric acid (Cretton et al. Simultaneous use of stavudine and didanosine is contraindicated due to the elevated frequency of mitochondrial toxicity. Co-prescription of stavudine and ribavirin has been related to an elevated threat of mitochondrial toxicities, especially lactic acidosis (Torriani et al. Co-prescription of brokers known to trigger peripheral neuropathy, significantly isoniazid and hydroxyurea, must be avoided because of their additive toxicity profiles.
Order abilify online
An open, randomised, 2-way crossover research to investigate the effect of darunavir/ritonavir on the pharmacokinetics of maraviroc in wholesome topics. Steady-state pharmacokinetic interaction study of atazanavir with ritonavir in wholesome subjects. Pharmacokinetic interplay between prasugrel and ritonavir in healthy volunteers. Increased danger of bleeding with the use of tipranavir boosted with ritonavir in haemophiliac patients. Saquinavir 500-mg filmcoated tablets reveal bioequivalence to saquinavir 200-mg exhausting capsules when boosted with twice day by day ritonavir in healthy volunteers. Pharmacokinetics and antiretroviral response to darunavir/ritonavir and etravirine mixture in patients with high-level viral resistance. Investigation of the interactions between methadone and elvitegravir-cobicistat in topics receiving continual methadone upkeep. Pharmacokinetics of cobicistatboosted elvitegravir administered in combination with methadone and buprenorphine/naloxone. Paper presented on the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco. An in vitro analysis of human cytochrome P450 3A4 inhibition by chosen industrial herbal extracts and tinctures. Effect of fosamprenavir/ ritonavir on the pharmacokinetics of single-dose olanzapine in healthy volunteers. Absence of opioid withdrawal symptoms in sufferers receiving methadone and the protease inhibitor lopinavir-ritonavir. Effect of ritonavir on the pharmacokinetics of the benzimidazoles albendazole and mebendazole: an interplay research in wholesome volunteers. Bioequivalence of two paediatric formulations vs grownup pill formulation of cobicistat. Paper introduced at the Conference on Retroviruses and Opportunistic Infections, Boston. Pharmacokinetics and safety of boosted-elvitegravir in subjects with hepatic impairment. Pharmacokinetics and drug interplay profile of cobicisat-boosted elvitegravir with carbamazepine. Paper presented at the10th Conference on Retroviruses and Opportunistic Infections, Boston. Preclinical assessment of the interplay between the antiretroviral medication, ritonavir and efavirenz, and the tyrosine kinase inhibitor erlotinib. Interaction between fosamprenavir, with and with out ritonavir, and nevirapine in human immunodeficiency virus-infected topics. Randomized trial to consider indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus sort 1-infected patients: the MaxCmin1 trial. Drug interactions between antiplatelet or novel oral anticoagulant medicines and antiretroviral medicines. Overview of drug-drug interactions with antiretroviral agents and different concomitant drugs. Steady state pharmacokinetic interactions between ritonavir, nelfinavir and the nelfinavir active metabolite M8. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct issue Xa inhibitor. The impact of cobicistat and cytochrome P250 2D6, 2B6 and P-glycoprotein utilizing phenotypic probes. Pharmacokinetic interplay between norgestimate/ethinyl estradiol and elvitegravir/cobicistat/ emtricitabine/tenofovir single pill regimen. Influence of separated and concomitant administration of ritonavir on the anticoagulant impact of dabigatran etexilate in wholesome volunteers. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib,and nilotinib.
Purchase on line abilify
In most cases (778; 91%), the severity of the poisoning was low, with high-grade toxicity being reported in solely eight cases. Symptoms and signs included oral irritation (19%), nausea (18%), vomiting (59%), abdominal cramps (4%), cough (4%), and seizures (3%). Chronic publicity to lindane has been related to hepatic injury and deranged liver operate, alopecia, and cardiotoxicity (Haustein, 1968; Schuttmann, 1968; Abdel Kader et al. Research on the potential carcinogenic potential of lindane in people has not established a particular carcinogenic impact (Fitzhugh et al. In a long-term cohort research involving greater than 140,000 people, of whom 1146 were exposed to lindane for therapeutic functions, forty three circumstances of most cancers have been noticed when only 30. In general, it can be said that evidence supporting an association between lindane exposure and cancer in people is weak and often confounded by other elements, similar to exposure of agricultural workers to different potential carcinogenic substances. Although other authors have suggested that lindane publicity is associated with a range of hematologic poisonous results, together with aplastic anemia, thrombocytopenia, leukopenia, pancytopenia, and leukemia (Jedlicka et al. In experimental animals, lindane exposure resulted in alterations within the degree of stress proteins and oxidative stress parameters in liver, heart, and testes (Junqueira et al. After topical software and oral ingestion, the exercise of liver enzymes (such as glutathioneS-transferase and cytochrome P450) was elevated in rats (Oesch et al. In the rat mannequin, lindane induced gamma-glutamyl-transpepitdase within the liver (Chandar and Nagarajan, 1984). Increased exercise of liver enzymes has additionally been reported after topical use of lindane in humans (Feldmann and Maibach, 1974; Chang et al. Lindane exposure also decreased humoral and cell-mediated immune responses in experimental animals (Saha and Banerjee, 1993; Banerjee et al. Apart from the toxic results of lindane on the central nervous system, in animal models it has been reported to cause depression of hematopoiesis, porphyria, toxic effects on the peripheral nervous system, testicular atrophy and hypertrophy, fatty degeneration and necrosis in kidneys, liver tumors, and fatty degeneration of the liver (Dallemagne et al. After topical therapy with 1% lindane, 50% of grownup rabbits confirmed central nervous system indicators; juvenile rabbits had convulsions, and half of them died (Hanig et al. Lindane also exhibited fetotoxicity in animal models, but no teratogenic effects have been noticed (Palmer et al. However, others think about lindane to be secure if remedy instructions are adopted strictly. Pediculosis In common, the pediculicidal and ovicidal exercise of lindane is lower than that of permethrin, pyrethrin, and malathion, significantly in more recent studies. This could mirror more appropriate research designs and/or lowered susceptibility of lice to lindane treatment. An overview of scientific research assessing the effectiveness of lindane in the remedy of head lice and body lice infestations is introduced in Table 211. Scabies Permethrin is considered the first-line therapy for scabies, but lindane continues to be prescribed in some nations due to its low price and availability (Bhalla and Thami, 2004). Similar to pediculosis resistance, resistance in scabies mite populations has been described. An overview of medical studies evaluating lindane for therapy of scabies is given in Table 211. Overall, lindane has been shown to be inferior to permethrin and ivermectin (Rezaee et al. Other makes use of When it was discovered, lindane was tested orally in people for the treatment of enterobiasis, however, owing to toxicity, this therapeutic indication was discarded instantly (Graeve and Herrnring, 1951; Solomon et al. It stays biologically energetic for approximately two years if saved unopened in a cool, shaded, and well-aired setting at 68�86�F. It is on the market in a variety of formulations, such as emulsifiable concentrate, wettable powder, dustable powder, and ultra�low quantity liquid formulations. It was one of the earliest organophosphate insecticides developed, being introduced into the United States in 1950 by the American Cyanamid Company (Brown et al. It is primarily used in opposition to agricultural pests, saved product bugs, and mosquitoes. Malathion is also recognized as carbophos (Russia), maldison (Argentina), and mercaptothion (Australia and New Zealand). Emerging resistance and cross-resistance the first instances of head lice resistant to malathion have been reported within the U. Since then, head lice resistance has elevated quickly (Witkowski and Charles Parish, 2002; Yoon et al. However, a susceptibility study confirmed that malathion 5% lotion retains helpful activity in areas with intermediate resistance, corresponding to Wales (Roberts et al.
Real Experiences: Customer Reviews on Abilify
Boss, 23 years: By ninety six weeks, 80% in the dolutegravir arm and 68% within the darunavir arm have been virologically suppressed.
Koraz, 22 years: In addition, additional selective stress with dolutegra vir in tissue culture led to the emergence of a subsequent mutation at place H51Y that modestly elevated fold change but significantly diminished viral integrase exercise and replicative health (Mespl�de et al.
Grubuz, 46 years: Stavudine is quickly absorbed after oral administration, with a imply bioavailability in adults, after a four mg/kg dose, starting from 70% to 90% (Dudley et al.
Ateras, 50 years: This has been recognized by many public health authorities who now advocate dimeticones as the therapy for pediculosis quite than permethrin, particularly in areas where permethrin resistance is common.
9 of 10 - Review by U. Ilja
Votes: 78 votes
Total customer reviews: 78